{ DOWNLOAD AS PDF }
About Authors:
Nehal C. Ghelani*, Krutika Bhalodiya, Ketan Dadhania, Shital Faldu
Department of Quality Assurance
Smt. R. D. Gardi B. Pharmacy College, Rajkot, Gujarat, India
*nehalghelani10@gmail.com
Abstract
UV Spectrophotometric method has been developed for simultaneous estimation of Olmesartan Medoxomil (OLME) and Cilnidipine (CILNI) in bulk drug and in laboratory mixture. This method utilizes methanol as a solvent and λmax of Olmesartan Medoxomil and Cilnidipine selected for analysis was found to be 241 nm and 253 nm respectively. Linearity was observed in the Olmesartan Medoxomil concentration range of 4-20μg/ml and Cilnidipine concentration range 2 -10 ug/ml (r2 = 0.998 and r2 0.999) of both drugs. The accuracy and precision were determined and found to comply with ICH guidelines. This method showed good reproducibility and recovery with % RSD in the desired range. The proposed methods can be successfully applied for the routine analysis of both the drugs. This method was simple, rapid, accurate, and sensitive.
INTRODUCTION
Olmesartan Medoxomil (OLME) (fig. 1a) is the chemically known as, 2,3-dihydroxy-2-butenyl4(1-hydroxy-1-methylethyl)-2-propyl-1-[p-(o-1H-tetrazol-ylphenyl)benzyl]imidazole carboxylate, cyclic 2,3-carbonate. Olmesartan is a prodrug that works by blocking the binding of angiotensin II to the AT1 receptors in vascular muscle; it is therefore independent of angiotensin II synthesis pathways, unlike ACE inhibitors. By blocking the binding rather than the synthesis of angiotensin II, olmesartan inhibits the negative regulatory feedback on renin secretion. As a result of this blockage, olmesartan reduces vasoconstriction and the secretion of aldosterone[1-3]. Cilnidipine(CILNE) (fig. 1b) the chemically known as, o3-(2-methoxyethyl) O5-[(E)-3-phenylprop-2-enyl]2,6-dimethyl-4-(3-nitrophenyl)-1,4dihydropyridine-3,5-dicarboxylate. Cilnidpiine is it reduces the incidense of pedal edema unlike amlodipine. Cilnidipine due to its blocking action at N-type calcium channel dilates both arteriole & venules as a result the pressure in the capillary bed is reduces. The accumulated fluid in the tissues flows back to veins & thus Cilnidipine minimizes the incidence of pedal edema[1-4]. Combination drug products of OLME and CILNI are widely marketed and used in the treatment of hypertension[5-7]. Several analytical methods like UV spectrophotometry, HPLC, HPTLC, UPLC have been reported for estimation of OLME & CILNI by single drug and also by combining with other drugs. However no methodhas been reported till date for the simultaneous estimation of OLME & CILNI using the UV spectrophotometric method. The present paper describes the development and validation of two analytical methods for simultaneous estimation of OLME & CILNI by UV spectrophotometry in tablet dosage form; the methods include simultaneous equation method & absorbance ratio method.[8-12] The proposed methods are optimized and validated as per the ICH guidelines [13-14].
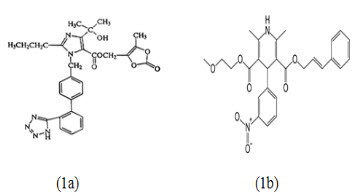
Fig. 1 Chemical structures of the analytes (1a) OLME & (1b) CILNI
MATERIALS AND METHODS
Instrumentation
Double beam UV-visible spectrophotometer (heλios Alpha, Model - V 7.09) having two matched quartz cells with 1 cm light path. An Electronic analytical balance (Contech, CA34 Model) was used in the study.
Material and reagent:
Double distilled water and Whatmann filter paper (0.45μm) were used for filtration. Active pharmaceutical ingredient (API) working standards of Olmesartan medoxomil (OLME), was obtained as gift sample from Intas Pharma limited, Ahemdabad, Indaa and Cilnidipine(CILNI) was obtained as gift sample from J. B. Chemicals, Surat, Gujarat, India and test samples (tablets with composition OLME-40 mg and CILNI – 10mg ) were procured from the local market.
Preparation of Standard Stock solution of OLME and CILNI:
Accurately weighed quantity of Olmesartan medoxomil 25 mg was transferred to 25 ml volumetric flask, dissolved in 10 ml of methanol and diluted up to mark with methanol to give a stock solution having strength of 1mg/ml.
Preparation of Working Standard Solution of OLME and CILNI:
100 µg/ml of OLME and CILNI solution were prepared by diluting 10 ml of stock solution to 100 ml with methanol in separate 100 ml volumetric flask. Suitable aliquots of this solution were diluted up to the mark with methanol to get the concentration range of 7,9,11,13,15 μg/ml for OLME and 3,4,5,6,7 μg/ml for CILNI.
(A) Preparation of Working Standard Solution OLME
100 mg/ml of OLME solution was prepared by diluting 1 ml of stock solution with methanol in 10 mlvolumetric flask up to the mark.
(B) Preparation of Standard Solution of Cilnidipine
Preparation of Standard Stock Solution of CILNI
Accurately weighed quantity of Cilnidipine 25 mg was transferred to 25 ml volumetric flask and make up to methanol and sonicate for 30 min for dissolving drug, to give a stock solution having strength of 1mg/ml.
Preparation of Working Standard Solution CILNI
100 mg/ml of CILNI solution was prepared by diluting 2.5 ml of stock solution with methanol in 25 ml volumetric flask up to the mark.
Procedure for Determination of Wavelength for Measurement
1.0 ml of working standard stock solution of OLME (100 mg/ml) and 1.0 ml of working standard stock solution of CILNI (100 mg/ml) were pipette out into two separate 10 ml volumetric flask and volume was adjusted to the mark with methanol to get 9mg/ml of OLME and 6 mg/ml of CILNI. Each solution was scanned between 200 - 400 nm against methanol as a reagent blank. Wavelengths were selected from the overlay spectra of OLME and CILNI.
Preparation of Calibration Curve
(A)Calibration Curve for OLME
Calibration curve for OLME consists of different concentrations of standard OLME solution ranging from 4-20 mg/ml. The solutions were prepared by pipetting out 0.7, 0.9, 1.1, 1.3, and 1.5 ml of the working standard solution of OLME (100 mg/ml) into series of 10 ml volumetric flasks and the volume was adjusted to mark with methanol. The absorbance of the solutions was measured at 253 nm and 241 nm against methanol as a reagent blank. Calibration curve was plotted at both wavelengths and two equations were formed using the specific absorbance (absorptivity).
(B) Calibration Curve for CILNI
Calibration curve for CILI consisted of different concentrations of standard CILNI solution ranging from 1-10 mg/ml. The solutions were prepared by pipetting out 0.3, 0.4, 0.5, 0.6 and 0.7 ml of the working standard solution of CILNI (100 mg/ml) into series of 10 ml volumetric flasks and the volume was adjusted to mark with methanol.
The absorbance of the solutions was measured at 253 nm and 241 nm against methanol as a reagent blank. Calibration curve was plotted at both wavelengths and two equations were formed using the specific absorbance (absorptivity).
Validation of proposed method
Parameters to be considered for the validation of method are:
Linearity and Range
The linearity response was determined by analyzing 5 independent levels of calibrationcurve in the range of 4 - 20 mg/ml and 1-10 mg/ml for OLME and CILNI respectively.
The calibration curve of absorbance vs. respective concentration was plotted and correlation coefficient and regression line equations for OLME and CILNI were calculated.
Precision
I) Repeatability
Aliquots of 1.1ml of working standard solution of OLME (100 mg/ml) were transferred to 10 ml volumetric flask. and Aliquots of 0.5 ml of working standard solution of CILNI (100 mg/ml) were respectively transferred to the same above 10 ml volumetric flask. The volume was adjusted up to mark to make 11 mg/ml of OLME and 5 mg/ml of CILNI solution was analysed 6 times on the same day spectrophotometry and % R.S.D. was calculated.
II) Intraday precision
Aliquots of 0.9, 1.1, and 1.3 ml of working standard solution of OLME (100 mg/ml) were transferred to a series of 10 ml volumetric flask. Aliquots of 0.4, 0.5 and 0.6 ml of working standard solution of CILNI (100 mg/ml) were respectively transferred to the same above series of 10 ml volumetric flask. The volume was adjusted up to mark with methanol to get 9, 11, and 13 mg/ml solution of OLME and 4, 5 and 6 mg/ml solution of CILNI. Solution was analysed3 times on the same day spectrophotometry and % R.S.D. was calculated.
III) Interday Precision
Aliquots of 0.9, 1.1, and 1.3 ml of working standard solution of OLME (100 mg/ml) were transferred to a series of 10 ml volumetric flask. Aliquots of 0.4, 0.5 and 0.6 ml of working standard solution of CILNI(100 mg/ml) were respectively transferred to the same above series of 10 ml volumetric flask. The volume was adjusted up to mark with methanol to get 9.0, 11, and 13 mg/ml solution of OLME and 4, 5 and 6 mg/ml solution of CILNI. Solution was analyzed 3 times on the 3 different day using spectrophotometry and % R.S.D. was calculated.
Accuracy
The accuracy of the method was performed by conducting the recovery studies (80, 100 and 120%) of pure drugs from marketed formulation, by standard addition method. The actual and measured concentrations were then compared.
LOD (Limit of Detection)
The LOD is estimated from the set of 6 calibration curves used to determine method linearity.
The LOD may be calculated as,
LOD = 3.3 s/S
where, s= The standard deviation of Y- intercept of 6 calibration curves.
S = The mean slope of the 6 calibration curves.
LOQ (Limit of Quantification)
The LOQ is estimated from the set of 6 calibration curves used to determine method linearity.
The LOQ may be calculated as,
LOD = 10 s/S
where, s= The standard deviation of Y- intercept of 6 calibration curves.
S = The mean slope of the 6 calibration curves.
Simultaneous Estimation of OLME And CILNI in Combined Dosage Form
The powder of twenty tablets were weighed. An accurately weighed quantity of the powder equivalent to about 40mg of OLME was taken in 10 ml volumetric flask and dissolved with methanol and further diluted upto the mark with same solvent. The solution was then filtered through the Whatmann filter paper No. 41. Necessary dilutions are made with methanol to give final concentration 40 µg/ml and 10 µg/ml of Olmesartan Medoxomil and Cilnidipine respectively. The solutions are then scanned between 200-400nm and absorbances are measured at respective wavelengths. The concentration of each drug was calculated using equation of straight line.
Absorbance of the resulting solution was measured at 253 nm and 241 nm against methanol. The concentration of OLME and CILNI can be obtained as
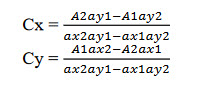
Where, A1, A2 are absorbance of mixture at 253 nm (l1) and 241 nm (l2) respectively,
ax1 and ax2 are absorptivity of OLME at l1 and l2 respectively,
ay1 and ay2 are absorptivity of CILNI at l1 and l2 respectively,
Cx and Cy are concentrations of OLME and CILNI respectively.
RESULTS AND DISCUSSION
Selection of Wavelength for Simultaneous Estimation of OLME and CILNI
To determine wavelength for measurement, standard spectra of OLME and CILNI were scanned between 200-400 nm against methanol. Absorbance maxima were obtained at 253 nm and at 241nm for OLME and CILNI respectively.
Method Validation:
The linearity range for OLME and CILNI were 7-15 μg/mL and 3-7 μg/mL respectively. Recovery studies was carried out by addition of standard drug solution to pre-analyzed dosage form solution at three different concentration levels (80%, 100% and 120%) taking into consideration percentage purity of added bulk drug sample. The results of the recovery studies are found to be satisfactory for OLME and CILNI and shown in Table 1 and 2 respectively.The result of assay procedure obtained was showed in Table 3. Summary of Other validation parameters including Repeatability, Intraday, Interday, LOD and LOQ were found to be satisfactory and are shown in Table 5.
CONCLUSION
The results obtained by applying the suggested procedures, it is proved that the proposed method is accurate, precise, simple, sensitive, selective and rapid and can be applied successfully in routine analysis for the estimation of OLMEand CILNIin their combined pharmaceutical dosage form. The developed method was validated as par ICH guidelines.
FIGURE AND TABLES
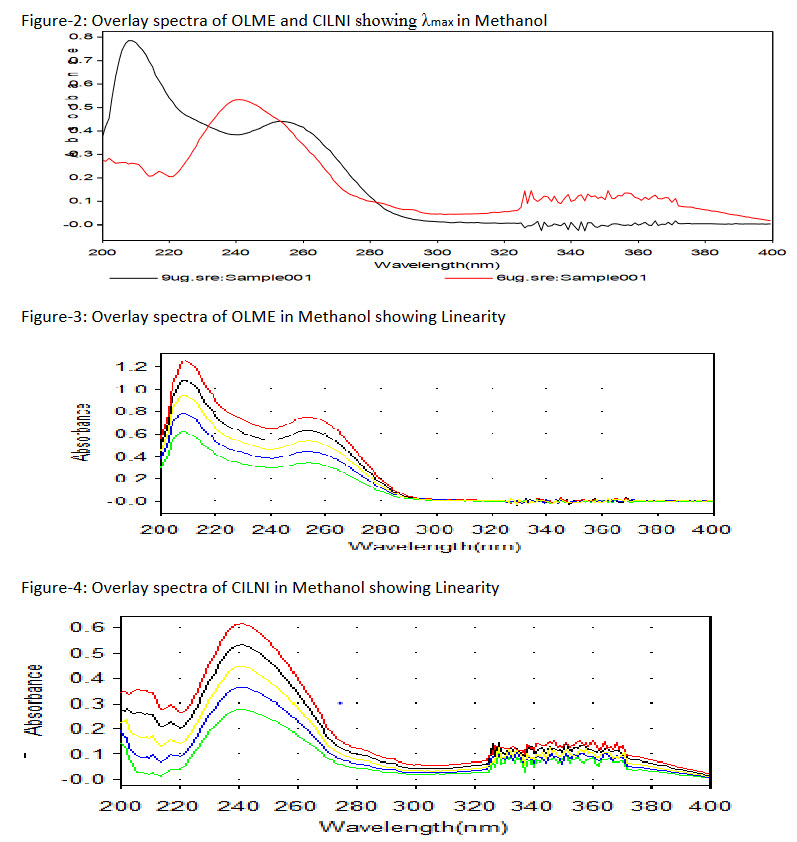
Table-1 Result of Recovery Studies for OLME in dosage form:
|
Amount of OLME in mixture (μg/ml) |
Amount of Std OLME added (μg/ml) |
Total amount of OLME (μg/ml) |
Total amount of OLME found (μg/ml) Mean* ± SD |
%Recovery |
|
|
|
|
|
|
|
11 |
8.8 |
19.8 |
19.37 ± 0.023 |
97.82 |
|
11 |
11 |
22 |
22.29 ± 0.001 |
101.31 |
|
11 |
13.2 |
24.2 |
24.31 ± 0.0020 |
100.45 |
[*=mean value of 3 determination]
Table-2 Result of Recovery Studies for CILNI in dosage form:
|
Amount of CILNI in Mixture (μg/ml) |
Amount of Std CILNI added (μg/ml) |
Total amount of CILNI (μg/ml) |
Total amount of CILNI found (μg/ml) Mean* ± SD |
%Recovery |
|
|
|
|
|
|
|
5 |
4 |
9 |
9.15±0.0151 |
101.66 |
|
5 |
5 |
10 |
10.10±0.00057 |
101 |
|
5 |
6 |
11 |
11.27±0.003055 |
102.45 |
[*=mean value of 3 determination]
Table-3: Analysis of OLME and CILNI in dosage form:
|
Tablet dosage form |
Label claim(mg) |
%Recovery ± SD (% of label claim*) |
||
|
OLME |
CILNI |
OLME |
CILNI |
|
|
|
40 mg |
10 mg |
98.75 ± 0.6186 |
101 ± 0.54477 |
[*=mean value of 5 determination]
Table-4: Regression Characteristics:
|
Characteristics |
OLME at 253 nm |
OLME at 241 nm |
CILNI at 253 nm |
CILNI at 241 nm |
|
Linearity (μg/ml) |
4-20 |
4-20 |
1-10 |
1-10 |
|
Regression Equation |
y = 0.050x - 0.014 |
y = 0.043x - 0.007 |
y = 0.071x + 0.015 |
y = 0.085x + 0.022 |
|
Slope |
0.050 |
0.043 |
0.071 |
0.085 |
|
r2 |
0.998 |
0.997 |
0.999 |
0.999 |
|
Intercept |
-0.014 |
0.007 |
0.015 |
0.022 |
|
S.D. of Intercept |
0.005508 |
0.005565 |
0.005292 |
0.002517 |
TABLE-5: VALIDATION PARAMETERS:
|
Parameters |
OLME at 253 nm |
OLME at 241nm |
CILNI at 253 nm |
CILNI at 241nm |
|
Repeatability(%RSD) (n=6) |
0.1289 |
0.1622 |
0.2656 |
0.1242 |
|
Precision (%RSD) |
|
|
|
|
|
Intra-day (n=3) |
0.3232-1.0129 |
0.2624-2.5147 |
0.2553-0.5998 |
0.2252-0.7112 |
|
Inter-day (n=3) |
0.3183-0.5510 |
0.400-3.6870 |
0.4174-0.6715 |
0.8049-1.3249 |
|
LOD (μg/ml) |
0.363 |
0.4226 |
0.245 |
0.097 |
|
LOQ (μg/ml) |
1.102 |
1.293 |
0.745 |
0.295 |
|
% Recovery (n=3) |
97.82%-101.31% |
|
|
100.86%-102.45% |
|
Assay (mean ± S.D.) (n=5) |
98.75±0.6186 |
|
|
101 ±0.54497 |
LOD: Limit of Detection, LOQ: Limit of Quantitation, R.S.D.: Relative standard deviation, S.D.: Standard deviation
ACKNOWLEDGEMENT
The authors are thankful to Smt. R. D. Gardi B. Pharmacy College, Rajkot, Gujarat, India for providing necessary facilities to carry out this work. We are also thankful to Intas Pharma, Ahemdabad &J. B. Chemicals, Surat for providing the free gift samples of Olmesartan Mediximil and Cilnidipine which was required for our research work.
REFERENCES:
(1) Tripathi, KD, “Essential of Medical Pharmacology”, 4th edition, new Delhi, Jaypee brothers, 2001, pp.521, 540
(2) The Merck Index – An Encyclopedia of Chemicals, Drugs and Biologicals, 13th Edn. Merck &Co., INC, 2001, pp 1223, 395
(3) En.wikipedia.org/Wiki/Olmesartan_medoxomil
(4) En.Wikipedia.org/Wiki/Cilnidipine
(5) drugaupdate.com/.../Olmesaetan%20 medoxomil
(6) Thedu.in/…/62697-olmark-40-mg-olmesartan-medoxomil
(7) drugaupdate.com/generic/view/1129
(8) Bhusari KP, Khedekar PB, Dhole SD and Banode VS, “Derivative and Q- analysis Spectrophotometric Methods for Estimation of Hydrochlorothiazide and OlmesartanMedoxomil in Tablets.”, Indian J Pharm Sci. 2009, 71(5): 505–508.
(9) SB, Wadkar SB, Raka KC and Chitlange SS, “Simultaneous Estimation of Amlodipine Besilate and Olmesartan Medoxomil in Pharmaceutical Dosage Form.”,Indian J Pharm Sci. 2009 71(5): 563–567.
(10) Jadhav JV, Burade KB, “An ecofriendly Simultaneous Estimation of Olmesartan Medoxomil ane Hydrochlorthazidein Pharmaceutical Dosage form by UV Visible Spectrophotometric Method.” , Der Pharma Chemica, 2013, 5(4):252-261.
(11) Jain PK, Jain AN, Maliwal DK, Jain VA, “Development & validation of spectrophotometric & for Estimation of Olmesartan Medoxomil in Tablet Dosage Form.” , International Journal of Pharma & Bio Sciences,2010, 1(2): pp1-7
(12) Haripriya MN, Antony NE, Jayasekhar PK,“Development and Validation of UV Spectrophtometric Method for the Simultaneous Estimation of Cilnidipine and Telmisartan in Tablet Dosage Form Utilising Simultaneous Equation and Absorbance ratio method.”, International journal of biological sciences,2013, 3(1):343-348.
(13) ICH guideline Q2 (R1). Validation of analytical procedures: text and methodology. Geneva: 1996.
(14) Beckett AH. And Stenlake JB. Practical Pharmaceutical Chemistry, part-II; 4th Edn; CBS Publisher and Distributors, New Delhi, 2007, pp 275-336.
REFERENCE ID: PHARMATUTOR-ART-2177
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 6 Received On: 04/04/2014; Accepted On: 09/04/2014; Published On: 01/06/2014 How to cite this article: NC Ghelani, K Bhalodiya, K Dadhania, S Faldu; Development and Validation of Spectrophotometric Method for Simultaneous Estimation of Olmesartan Medoxomil and Cilnidipine by Simultaneous Equation Method; PharmaTutor; 2014; 2(6); 160-166 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









