About Authors:
Gedam V. K., Shrivastav S. R., Raut N.A.
Department of Pharmaceutical Sciences RTM, Nagpur University, Amravati Road,
Nagpur- 440033.
*vkgedam8@hotmail.com
ABSTRACT
The present research work discusses the development of a UV spectrophotometric method for Dronedarone hydrochloride. Simple, accurate, cost efficient and reproducible spectrophotometric method has been developed for the estimation of Dronedarone hydrochloride in bulk and pharmaceutical dosage form. UV spectrophotometric method, which is based on measurement at maximum wavelength (λmax) at 290nm using methanol as a solvent. The percentage recovery of Dronedarone hydrochloride ranged from 98.48 to 98.96% in pharmaceutical dosage form. Beers law was obeyed in the concentration range of 2-30μg/ml having line equation y = 0.0397x - 0.0060 with correlation coefficient of 0.9998. Results of the analysis were validated statistically and by recovery study.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1621
INTRODUCTION:
Analysis is an important component in the formulation development of any drug molecule. It becomes essential to develop a simple, sensitive, accurate, precise, reproducible method for the estimation of drug samples. Literature review for Dronedarone hydrocloride analysis revealed several methods based on different technique such as LC-MS and HPLC with UV detection for quantification of their active metabolites in human plasma and myocardium. However there is no method reported for the detection of Dronedarone hydrochloride in bulk and pharmaceutical formulation by UV spectrophotometry with such parameters. Our main objective is development and validation of UV spectrophotometric method as per ICH guideline.[1,2]
DRUG PROFILE[2]:
Dronedarone hydrochloride:-
Dronedarone hydrochloride is new antiarrhythmic drug used in the treatment of atrial fibrillation and atrial flutter. It is available in the strength of 400 mg tablets.
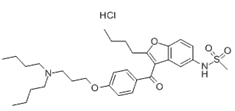
Fig.1. Dronedarone hydrochloride
[adsense:468x15:2204050025]
Chemical name: N-[2-butyl-3-[4-[3-(dibutylamino) propoxy] benzoyl]-1benzofuran-5- Eel] methanesulfonamide hydrochloride
CAS Number: 141626-36-0
Molecular formula: C31H44N2O5S . HCl
Molecular weight: 556.758g/mol
Physical Appearance: White solid
Melting point: 140-145 °C
Solubility: Soluble inmethanol and acetonitrile, but insoluble in water
MATERIALS AND METHODS:
Instrument used were Shimadzu UV-1700, Shimadzu UV PC-2401 UV/Visible Spectrophotometer and Metteler Toledo AG-135 analytical balance. Dronedarone hydrochloride pure drug was obtained from Emcure Pharma, Jammu. As gift sample with 99.9% w/w assay value and was used without further purification. All chemicals and reagents used were of analytical grade. Dronedarone hydrochloride tablets (Dilsave 400 mg) were purchased from local market.
Preparation of standard stock solution: Standard stock solution of Dronedarone hydrochloride was prepared by dissolving 5 mg in 10ml pure methanol in 50ml volumetric flask and volume was made up to mark with methanol to get stock solution of 100µg/ml concentration. For obtaining clear solution, solution was ultrasonicated for 15 minutes.
Preparation of working standard solution: 1ml of standard stock solution was pipetted out to 10 ml volumetric flask and volume was made up to the mark with methanol to get concentration of 10µg/ml.
RESULTS AND DISCUSSION:
A. Determination of Working Wave Length (λmax):
In order to ascertain the wavelength of maximum absorption (λ max) of the drug, working standard solution of the drug (10μg/ml) in methanol was scanned using UV-VIS spectrophotometer within the wavelength region of 200 – 400 nm against solvent blank. The resulting spectra are shown in figure below and the absorption curve shows characteristic absorption maxima at 290nm for Dronedarone hydrochloride.
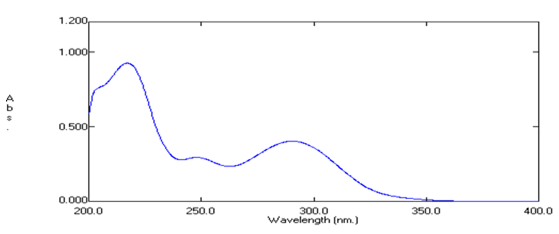
Fig.2: UV-VIS spectrum of Dronedarone hydrochloride
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
B. Preparation of Calibration Curve:
For preparation of calibration curve of Dronedarone, a stock solution of 100µg/ml was prepared. From it different concentrations ranging from 2 – 30 µg/ml was prepared. Then the samples were scanned through UV-VIS Spectrophotometer at 290 nm and their absorbance’s were noted, which were given in the table below. A calibration curve was plotted using those readings taking Concentration on X-axis and Absorbance on Y-axis.
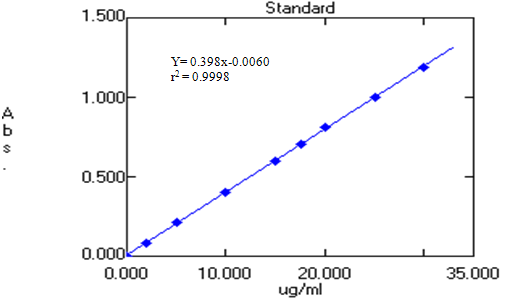
Fig: 3. Calibration Curve of Dronedarone hydrochloride
From the calibration curve it was found that it shows linearity in the range of 2– 30µg/ml with Correlation coefficient 0.9998.
Table.No.1: Calibration Curve parameter
|
Concentration (µg/ml) |
Absorbance at 290nm |
|
2 |
0.085 |
|
5 |
0.210 |
|
10 |
0.399 |
|
15 |
0.598 |
|
17.5 |
0.707 |
|
20 |
0.810 |
|
25 |
0.998 |
|
30 |
1.191 |
C. Determination of Absorptivity Value, A (1%, 1cm) of Dronedarone hydrochloride at selected wavelength:
Accurately measured 1 ml of stock solution was added to 10 ml volumetric flask and volume was made upto the mark with methanol to get final concentration of 10µg/ml of Dronedarone five dilutions. Absorbance for each dilution was measured at 290nm against solvents blank and aborptivity values were calculated.
Table No. 2. A (1%, 1cm) of Dronedarone hydrochloride at 290 nm
|
Sr.No |
A(1%, 1 cm)* |
|
1 |
393.2 |
|
2 |
393.4 |
|
3 |
394.2 |
|
4 |
393.6 |
|
5 |
393.2 |
|
MEAN |
393.52 |
|
S.D |
0.4147 |
D. Estimation of Dronedarone hydrochloride in Tablet Formulation:
The proposed method was applied to analyse commercially available Dronedarone hydrochloride tablet. Ten tablets were weighed and powdered. The amount of tablet powder equivalent to 5 mg of Dronedarone was weighed accurately and transfer to 50ml volumetric flask then 10 ml methanol was added and kept for 15 min for ultrasonication, and the volume was made up to the mark with methanol. The solution was then filtered through Whattman filter paper, 1ml of the filtrate was diluted suitably with solvent up to 10 ml to get the solution of 10µg/ml concentration .The absorbance was measured against solution blank. The % estimation of Dronedarone hydrochloride was calculated by using absorptivity value A (1%, 1cm) i.e., 393.52 (MethodA) and by direct Comparison with standard (MethodB).
Table No. 3 Results of estimation of Dronedarone hydrochloride in Tablets
|
Dilsave tablet(Avg. Wt. 670.2 mg for 400 mg of Dronedarone hydrochloride hydrochloride) |
||||||
|
Sr. No.
|
Weight taken (mg)
|
Standard Concentration (µg/ml) 10 |
Absorbance at 290 nm |
% Drug Estimation
|
||
|
Standard |
Sample |
Method A* |
Method B * |
|||
|
2 |
8.4 |
10 |
0.395 |
0.398 |
100.86 |
100.48 |
|
3 |
8.5 |
10 |
0.395 |
0.399 |
99.93 |
98.72 |
|
4 |
8.4 |
10 |
0.395 |
0.395 |
100.10 |
99.73 |
|
5 |
8.6 |
10 |
0.395 |
0.401 |
99.26 |
98.89 |
|
|
8.4 |
|
0.395 |
0.391 |
99.09 |
99.55 |
|
|
Mean |
99.84 |
99.47 |
|||
|
± S.D |
0.7096 |
0.7058 |
||||
|
RSD |
0.71% |
0.70% |
||||
*Each value is mean of five observations
E. METHOD VALIDATION (as per ICH guidelines) [1,4]:
1. Accuracy:
Sample Solutions were prepared at by adding 80%, 100% and 120% pure drug as per the test method dilution was made as according to and absorbance of each solutions were taken .The recovery result showed that the proposed method has an acceptable level of accuracy for Dronedarone hydrochloride which is between a mean value 98.48 % to 98.96 % for the test concentrations ranging from 80% to 120%.
Table No. 4. Results of Recovery studies
|
Dilsave tablet(Avg. Wt. 670.2 mg for 400 mg of Dronedarone hydrochloride) |
||||||
|
Sr. No |
Sample weight + pure drug added |
Std. Conc. (µg/ml)
|
Absorbance at 290 nm |
% Drug Estimation
|
||
|
Standard |
Sample |
Method A* |
Method B * |
|||
|
1 |
8.4+ 4 |
10 |
0.395 |
0.697 |
98.13 |
97.76 |
|
2 |
8.4+ 5 |
10 |
0.395 |
0.784 |
99.34 |
98.59 |
|
3 |
8.4+ 6 |
10 |
0.395 |
0.863 |
99.41 |
99.04 |
|
|
Mean |
98.96 |
98.48 |
|||
|
± S.D |
0.7196 |
0.6331 |
||||
|
RSD |
0.73% |
0.64% |
||||
* Each value is mean of five observations
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2. Precision
The tablet samples of dronedarone hydrochloride and working standard of concentration 10µg/ml was prepared and precision studies analysed by using different analysts , different days and different instruments.
A. Different analyst
Table No. 5.1 Results of precision studies using different analysts
|
Sr . No. |
Different Analysts |
% Drug Estimation
|
|
|
Method A* |
Method B * |
||
|
1 |
Analyst 1 |
99.02 |
98.64 |
|
2 |
Analyst 2 |
99.85 |
99.47 |
|
3 |
Analyst 3 |
100.36 |
99.98 |
|
|
Mean |
99.74 |
99.36 |
|
± S.D |
0.6763 |
0.6763 |
|
|
R.S.D |
0.68% |
0.68% |
|
* Each value is mean of five observations
B. Different instruments
Table No. 5.2 Results of precision studies using different instruments
|
Sr. No |
Different Instruments |
Observations |
% Drug Estimation
|
|
|
Method A* |
Method B * |
|||
|
1 |
UV-1701 |
1 |
98.92 |
98.55 |
|
|
|
2 |
100.43 |
100.05 |
|
2 |
UVPC-2401 |
1 |
99.26 |
98.89 |
|
|
|
2 |
99.66 |
99.89 |
|
|
Mean |
99.56 |
99.21 |
|
|
± S.D |
0.6496 |
0.6512 |
||
|
R.S.D |
0.65% |
0.66% |
||
* Each value is mean of five observations
C. Different days
Table No. 5.3 Results of precision studies in different days
|
Sr. No |
Observations |
% Drug Estimation |
|||
|
Interday |
Intraday |
||||
|
Method A* |
Method B * |
Method A* |
Method B * |
||
|
1 |
I |
100.10 |
99.73 |
99.85 |
99.47 |
|
2 |
II |
100.68 |
100.30 |
99.93 |
99.55 |
|
3 |
III |
99.34 |
99.97 |
99.17 |
98.80 |
|
|
Mean |
100.04 |
100.01 |
99.65 |
99.27 |
|
± S.D |
0.6720 |
0.2861 |
0.4176 |
0.4188 |
|
|
R.S.D |
0.67% |
0.29% |
0.42% |
0.41% |
|
* Each value is mean of five observations
3. Specificity:
An accurately weighed tablet powdered equivalent to 5 mg was weighed in six different 50 ml volumetric flask. All were stored at 24 hrs under following different condition below and after 24 hrs same procedure followed as per test method to get final concentration of 10µg/ml. The absorbance of these solutions was measured at 290nm against solvent blank
1. 1 Normal,
2. At 50°C after addition of 1.0ml of 0.1 N Hcl,
3. At 50°C after addition of 1.0ml of 0.1 N NaOH
4. At 50°C after addition of 1.0ml of 3% H2O2
5. At 60°C(heat),
6. InUV Chamber
Results of specificity showsdrug estimation range of 99.013% to 99.45%
4. Linearity Range:
The linearity of the response of the drug was verified at 2 to 30 µg/mlconcentrations. The calibration curve was obtained by plotting the absorbance versus the concentration data and was treated by linear regression analysis .The equation of the calibration curve of Dronedarone hydrochloride obtained was y = 0.0398-0.060, having slope 0.0398 with a correlation coefficient 0.9999, the calibration curve was found to be linear in aforementioned concentrations.
5. Robustness:
Change in wavelength (± 2 nm):
Table No. 6 Results of Robustness study
|
Sr . No. |
Wavelength (± 2 nm) |
% Drug Estimation
|
|
|
Method A* |
Method B * |
||
|
1 |
288 nm |
99.51 |
99.13 |
|
99.26 |
98.89 |
||
|
2 |
290 nm |
99.26 |
98.89 |
|
100.50 |
99.13 |
||
|
3 |
292 nm |
100.74 |
100.37 |
|
99.26 |
98.89 |
||
|
|
Mean |
99.79 |
99.42 |
|
± S.D |
0.6515 |
0.6519 |
|
|
R.S.D |
0.66% |
0.65% |
|
*Each value is mean of five observations
CONCLUSION:
The developed method was found to be simple, sensitive, accurate, precise, reproducible, and can be used for routine quality control analysis of Dronedarone hydrochloride in bulk and pharmaceutical formulation.
ACKNOWLEDGEMENT:
We would like to thanks to our Head of the deparment, Departmnt of Pharmaceutical Sciences , Nagpur University Campus for providing all the research facilities. We are also thankful to Emcure Pharma, Jammu. for giving us gift sampleof Dronedarone hydrochloride drug.
REFERENCES:
1. International Conference on harmonization, “ICH Q2 (R1) Validation of Analytical Procedures: Text Methodology,” November 2005, 1-13.
2. Dorian P. Clinical Pharmacology of Dronedarone: Implications for the Therapy of Atrial Fibrillation. J. Cardiovasc. Pharmacol. Ther. 2010, 15(4 Suppl):15S-8S.
3. Khopkar SM. “Basic Concepts of Analytical Chemistry” 3rd ed., New Age International Publisher. 2008; 277-278.
4. Clark BJ., Frost T., Russell MA “UV Spectroscopy Techniques, instrumentation and data handling.” Springer, 1993, 1 - 164.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









