About Author:
Deepa yadav
M.Pharm
Rameshwaram institute of Technology and management, Lucknow
deepa.yadav.amethi@gmail.com
ABSTRACT:
Dendrimers have emerged as one of the most interesting themes for researchers as a result of unique functional architecture and macromolecular characteristics dendrimer, have attracted great attention in terms of biomedical applications. Although the PAMAMdendrimer has already been tested as a carrier for drugs and genes and as a contrast agent for bioimaging . This mini review highlights issues associated with the use of dendrimers as drug delivery vehicles. This article provides an insight into the structure, synthesis, properties,types and the applications of dendrimers in the bio-medical field.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1427
DENDRIMER
In these formative years of nanotechnology, one of the most frequently seen names in the scientific literature is a class of polymerized macromolecules that carry the potential to provide the most exquisitely tailored forms and functions ever realized outside of nature. These polymerized macromolecules named as “dendrimers” are visualized as the polymers of 21st century. The term "dendrimer" is derived from the Greek words dendron, meaning tree and meros, meaning part. Dendrimers were introduced in 1980’s by Donald A. Tomalia, scientific director of the Center for Biologic Nanotechnology at the University of Michigan. Ideally, these are perfectly monodisperse macromolecules with a regular and highly branched three-dimensional architecture. Think of a tree in which each of its branches divides into two new branches after a certain length. This continues repeatedly until the branches become so densely packed that the canopy forms a globe. In a dendrimer, the branches are interlinked polymerized chains of molecules, each of which generates new chains, all of which converge to a single focal point or core .[1]
In 1978, Fritz Vogtle and co-workers, introduced dendrimer chemistry[2] and in 1985, Donald A. Tomalia, synthesized the first family of dendrimers[3]. Dendrimers are repeatedly branched roughly spherical large molecules and possess well defined chemical structures[4]. The word dendrimer comes from a Greek word which means to “tree”. At the same time, Newkome’s group independently reported synthesis of similar macromolecules. They called them arborols from the Latin word ‘arbor’ also meaning a tree[5].The other synonyms for dendrimer include cascade molecules. It is a highly branched synthetic polymer and consists of a monomer unit attached core, where a, leading to a monodisperse, treelike, star-shaped or generational structure with precise molecular weights, diameters in the 2 to 10 nm range size, its unique architectural design, high degree of branching, multivalency, globular structure and representative of a new segment of polymer science, often been referred to as the “Polymers of the 21st century”. Poor solubility, bioavailability, permeability, biocompatibility and toxicity can be overcome by dendrimers. Recent successes in simplifying and optimizing the synthesis of dendrimers provide a large variety of structures with reduced cost of their production. Dendritic polymers or dendrimers provide a route to create very well-defined nanostructures suitable for drug solubilisation applications, delivery of oligonucletide, targeting drug at specific receptor site, and ability to act as carrier for the development of drug delivery system. Dendrimers are being considered as additives in several routes of administration, including intravenous, oral, transdermal, pulmonary and ocular.[6]
STRUCTURE OF DENDRIMERS
A dendrimer is typically symmetric around the core , and often develops a three dimensional morphology .In the view of polymer chemistry dendrimeres are perfect monodisperse macro molecules with regular highly branched three dimensional structures and consist of three architectural components like core,branches and end groups.[7,8]Dendrimers of lower generations (0, 1, and 2) have highly asymmetric shape and possess more open structures as compared to higher generation dendrimers. As the chains growing from the core molecule become longer and more branched (in 4 and higher generations) dendrimers adopt a globular structure[9]. Dendrimers become densely packed as they extend out to the periphery, which forms a closed membrane-like structure. When a critical branched state is reached dendrimers cannot grow because of a lack of space. This is called the ‘starburst effect[10]. For PAMAM dendrimer synthesis it is observed after tenth generation. The rate of reaction drops suddenly and further reactions of the end groups cannot occur. The tenth generation PAMAM contains 6141 monomer units and has a diameter of about 124 Å [11]The increasing branch density withgeneration is also believed to have striking effects on the structure of dendrimers. They are characterised by the presence of internal cavities and by a large number of reactive end groups . Dendritic copolymers are a specific group of dendrimers. There are two different types of copolymer
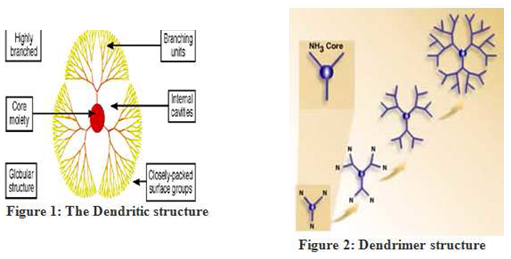
Segment-block dendrimers are built with dendritic segments ofdifferent constitution. They are obtainedby attaching different wedges to onepolyfunctional core molecule.
Layer-block dendrimers consist of concentric spheres of differing chemistry.They are the result of placing concentric layers around the central core. Hawker and Fréchet[12] synthesised a segment- block dendrimer which had one ether-linked segment and two ester-linked segments. They also synthesised a layer-block dendrimer. The inner two generations were ester-linked and the outer three etherlinked. The multi-step synthesis of large quantities of higher generation dendrimers requires a great effort. This was the reason why Zimmerman’s group applied the concept of self-assembly to dendrimer Synthesis[13]. They prepared a wedgelike molecule with adendritic tail in such a manner that six wedge-shaped subunits could self-assemble to form a cylindrical aggregate. This hexameric aggregate is about 9 nm in diameter and 2 nm thick. It has a large cavity in the centre. The six wedges are held together by hydrogen bonds between carboxylic acid groups and stabilised by Vander Waals interactions. However, the stability of the hexamer is affected by many factors. The aggregate starts to break up into monomers when the solution is diluted, when the aggregate is placed in a polar solvent like tetrahydrofuran (THF), and when the temperature is high. The hexamer’s limited stability is due to its noncovalent nature.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PROPERTIES OF DENDRIMER
Monodispersity
Dendrimers are the class of dendritic polymers that can be constructed with a well-defined molecular structure, i.e. being mono-disperse, unlike to linear polymers. Monodispersity offers researchers the possibility to work with a tool for welldefined scalable size.
Nanoscale size and shape
These fundamental properties have in fact lead to their commercial use for gene therapy, immunodiagnostics and variety of other biological applications.
Polyvalency
Polyvalency shows the outward presentation of reactive groups on the dendrimer nanostructure exterior. This creates more connections between surfaces and bulk materials for applications such as adhesives, surface coatings, or polymer cross-linking. The product, a topical vaginal microbicide called Vivagel™, prevents infection by HIV and other sexually transmitted diseases during intercourse takes advantage of dendrimers polyvalent properties.
Physicochemical properties of dendrimers
The use of dendrimers as protein mimics has been encouraged scientists to investigate the physicochemical properties of dendrimers in comparison to proteins shows that dendrimers, similar to protein, can adapt “native” (e.g. tighter) or “denaturated” (e.g.extended) conformations dependent on the polarity, ionic strength and pH of the solvent.
Biocompatibility of dendrimers
In order to utilize dendrimers as biological agents, they have to fulfill certain biological demands. The dendrimer should be: nontoxic, non-immunogenic, able to cross biobarriers (biopermeable), able to stay in circulation for the time needed to have a clinical effect and able to target to specific structures. The cytotoxicity of dendrimers has been primarily evaluated in vitro;
however, a few in vivo studies have been published [14,15]. Dendrimers with positively charged surface groups are prone to destabilize cell membranes and cause cell lysis. For example, in vitro cytotoxicity, IC50 measurements (i.e., the concentration where 50% of cell lysis is observed) for poly (amidoamine) PAMAM dendrimers with amino surface revealed significant cytotoxicity on human intestinal adenocarcinoma, Caco-2 cells. Comparative toxicity studies on anionic (carboxylate-terminated) and cationic (amino-terminated) PAMAM dendrimers using Caco-2 cells have shown significantly lower cytotoxicity of the anionic compounds [16]. Furthermore, the cytotoxicity was found to be generation dependent with higher generation dendrimers being the most toxic,[17] Some recent studies have shown that amino-terminated PAMAM dendrimers exhibit lower toxicity than more flexible linear polymers carrying amine groups, perhaps due to lower adherence of the rigid globular dendrimers to cellular surfaces. The degree of substitution as well as the type of amine functionality is important, with primary amines being more toxic than secondary or tertiary amines.Lower-generation PAMAM dendrimers possessing carboxylate surface groups show neither haemato-toxicity nor cytotoxicity at concentrations up to 2 mg/ml [18].
Immunogenicity
Immunogenicity is one of the crucial biological properties of the dendrimers. Studies performed on unmodified aminoterminated PAMAM dendrimers showing no or only weak immunogenicity of the G3–G7 Many dendrimer syntheses rely upon traditional reactions, such as the Michael reaction or the Williamson ether synthesis whilst others involve the use of modern dendrimers. However, later studies showed some immunogenicity of these dendrimers and found that modification of aminoterminated PAMAM dendrimers with polyethylene glycol (PEG) chains reduces immunogenicity and gives longer lifetime in the blood stream in comparison to unmodified dendrimers [19]
Advantages of Dendrimer
.Dendrimers offers various advantages over other polymers:
(1) Dendrimers have nanoscopic particle size range from 1- 100 nm, which makes them less susceptible for reticulum endothelium uptake.
(2) They have lower polydispersity index, due to stringent control during synthesis. As the density of branches increases the outer most branches arrange themselves surrounding a lower density core in the form of spheres and outer surface density is more and most of the space remains hollow towards core. This region can be utilized for drug entrapment.
(3) Multiple functional groups are present on outer surface of dendrimers, which can be used to attach vector devices for targeting to particular site in the body.
(4) Dendrimers can be modified as stimuli responsive to release drug.
(5) Dendrimers might show an enhanced permeability and retention effect which allows them to target tumour cells more effectively than small molecules.
(6) They can be synthesized and designed for specific applications. Due to their feasible topology, functionality and dimensions, they are ideal drug delivery systems; and also, their size is very close to various important biological polymers and assemblies such as DNA and proteins whichare physiologically ideal.[20,21,22]
TYPES OF Dendrimer
(1) Radially layered poly (amidoamine-organosilicon) dendrimers (PAMAMOS)
In 1990, Dr. Petar Dvornic and his colleagues at Michigan Molecular Institute, discovered this unique first commercial silicon containing dendrimers. Consist of hydrophilic, nucleophilic polyamidoamine (PAMAM) interiors and hydrophobic organosilicon (OS) exteriors. Excellent its networks regularity and ability to complex and encapsulate various guest species offer unprecedented potentials for new applications in nanolithography, electronics, photonics, chemical catalysis etc.and useful precursors for the preparation of honeycomblike networks with nanoscopic PAMAM and OS domains.[23,24]
(2) Poly (amidoamine) dendrimers (PAMAM)
Synthesized by the divergent method, starting from initiator core reagents like ammonia or ethylenediamine. When looking at the structure of the high-generation in two-dimensions, star like pattern observed. They are commercially available as methanol solutions and in generation G 0-10 with 5 different core type and 10 functional surface groups.[25,26]
(3) Poly (Propylene Imine) dendrimers (PPI)
Poly (Propylene Imine) dendrimers (PPI) generally having poly-alkyl amines as end groups, and numerous tertiary tris-propylene amines present in interior portion. It commercially available up to G5, and wide applications in material science as well as in biology.[27]PPI dendrimers are available as AstramolTM.
(4) Chiral dendrimers
The chirality in these dendrimers is based upon the construction of constitutionally different but chemically similar branches to chiral core. Their potential use as chiral hosts for enantiomeric resolutions and as chiral catalysts for asymmetric synthesis.[28]
(5) Liquid crystalline dendrimers
A highly-branched oligomer or polymer of dendritic structure containing mesogenic groups that can display mesophase behaviour. They consist of mesogenic (liq. crystalline) monomers e.g. mesogen functionalized carbosilane dendrimers.
(6) Tecto dendrimer
Tecto Dendrimer are composed of a core dendrimer,perform varied functions ranging from diseased cellrecognition, diagnosis of disease state drug delivery,reporting location to reporting outcomes of therapy.
(7) Hybrid dendrimers
Hybrid dendrimers are hybrids (block or graft polymers) of dendritic and linear polymers. Obtained by complete monofunctionalization of the peripheral amines of a "zero-generation" polyethyleneimine dendrimer, provide structurally diverse lamellar, columnar, and cubic selforganized lattices that are less readily available from other modified dendritic structures.
(8) Multilingual Dendrimers
Multilingual Dendrimers contains multiple copies of a particular functional group on the surface.
(9) Micellar Dendrimers
Micellar dendrimers are unimolecular water solublhyper branched polyphenylenes micelles.
(10) Amphiphilic Dendrimers
Amphiphilic dendrimers are built with two segregated sites of chain end, one half is electron withdrawing andthe other half is electron donating.
(11) Peptide dendrimers
Multiple Antigen Peptide dendrimers is a dendron-like molecular construct, use in biological applications, e.g. vaccine and diagnostic research. Peptide dendrimers can be used as drug delivery, contrast agents for magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), fluorogenic imaging and sero diagnosis.[29]
(12) Frechet-Type Dendrimers
Frechet-Type Dendrimers have carboxylic acid groups as surface groups, serving as a good anchoring point for further surface functionalisation, and as polar surface groups to increase the solubility of this hydrophobic dendrimer type in polar solvents or aqueous media.[30.31]
SYNTHESIS OF DENDRIMER
(1) Divergent growth method
This method was introduced by Tomalia. In this method growth of dendrimers originates from a core site. The core is reacted with two or more moles of reagent containing at least two protecting branching sites, followed by removal of the protecting groups, lead to the first generation dendrimers. This process is repeated until the dendrimer of the described size is obtained. By this approach the first synthesized dendrimers were polyamidoamines (PAMAMs), also known as starbust dendrimers[32]
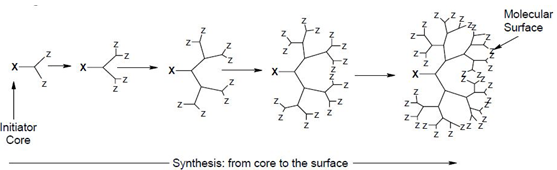
Divergent method for the synthesis of dendrimer
(2) Convergent Dendrimer Growth
Convergent dendrimer growth begins at what will end up being the surface of the dendrimer, and works inwards by gradually linking surface units together with more. When the growing wedges are large enough, several are attached to a suitable core to give a complete dendrimer. convergent growth method has several advantages like relatively easy to purify the desired product, occurrence of defects in the final structure is minimised, does not allow the formation of high generation dendrimer because stearic problems occur in the reactions of the dendrons and the core molecule.[33]
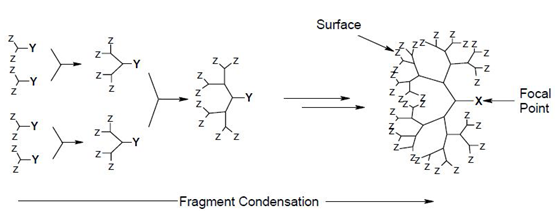
Convergent method for synthesis of dendrimer
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Characterizations of dendrimer by various methods
METHODS FOR CHARACTERIZATION OF DENDRITIC POLYMER
(A) Spectroscopy and spectrometry methods [34-39] most widely used for dendrimers Characterization like
Nuclear Magnetic Resonance (NMR) Analysis in step by step synthesis of Dendrimer. To Probe the Size, Morphology and Dynamics of Dendrimers for organic dendrimers such as PPI etc.
Ultra-violet-visible spectroscopy (UV-VIS) Used to monitor synthesis of dendrimers. The intensity of the absorption band is essentially proportional to the number of chromophoric units.
Infra red spectroscopy (IR) for routine analysis of the chemical transformations occurring at the surface of dendrimers.
Near infra red spectroscopy Used to characterize delocalize π-π stacking interaction between end groups of modified PANAM.
Fluorescence The high sensitivity of fluorescence has been used to quantify defects during the synthesis of dendrimers.
Raman spectroscopy gave relevant information about the degree of cyclodehydrogenation of polyphenylene dendrimers, and the characterization of PPI and dendrimers.
Mass spectroscopy Chemical ionization or fast atom phosphorus bombardment can be used only for the characterization of small dendrimers whose mass is below 3000 Da. Electrospray ionization can be used for dendrimers able to form stable multicharged species.
X-ray diffraction (XRD) This technique should allow precise determination of the chemical composition, structure, size and shape of Dendrimer.
(B) Scattering techniques[[40-43]
Small angleX-ray scattering (SAXS) gives information about their average radius of gyration (Rg) in solution. The intensity of the scattering as a function of angle also provides information on the arrangement of polymer segments, hence on the segment density distribution within the molecule.
Small angle neutron scattering (SANS) gives access to the radius of gyration, but may also reveal more accurate information than SAXS about the internal structure of the entire dendrimer. The location of the end groups has also been determined by SANS experiments conducted with PAMAM dendrimers and PPI dendrimers having labeled (deuterated) or unlabelled end groups.
Laser light scattering (LLS) to determine the hydrodynamic radius of dendrimers. Dynamic LLS is mainly used for the detection of aggregates.
(C) Microscopy methods[44,45]
Transmission microscopy Electron or light produce images that amplify the original, with a resolution ultimately limited by the wavelength of the source
Scanning microscopy The image is produced by touch contact Q at a few angstroms of a sensitive canilever arm with sample. Ex. Atomic force microscopy.
(D) Size exclusive chromatography[46] allows the separation of molecules according to size.
(E) Electrical techniques[47,48,49]
Electron paramagnetic resonance (EPR) Quantitative determination of the substitution efficiency on the surface of PANAM dendrimers.
Electrochemistry gives information about the possibility of interaction of electroactive end groups.
Electrophoresis used for the assessment of purify and homogeneity of several type of water soluble dendrimers.
(F) Rheology and Physical properties[50-53]
Intrinsic viscosity used as analytical probe of the morphological structure of dendrimers.
Differential scanning calorimetry (DSC) used to detect the glass transition temperature which depends on the moleculer weight, entangment and chain composition of polymers.
Dielectric spectroscopy (DS) Gives information about molecular dynamic processes (α-, β)
(G) Miscellaneous[54-56]
X-ray Photoelectron Spectroscopy (XPS) chemical composition of dendrimers such as poly (aryl ether) dendrons or PMMH dendrimers has been also obtained using XPS, even if this technique is most generally used for the characterization of layers.
Sedimentation for lactosylated PAMAM dendrimers, measurements of dipole moments for PMMH dendrimer.
Titrimetry to determine the number of NH2 end groups of PAMAM dendrimers
APPLICATION OF DENDRIMER
Pharmaceutical application
Dendrimer in ocular drug delivery
Ideal ocular drug-delivery systems should benonirritating, sterile, isotonic, biocompatible, does not run out from the eye and biodegradable.Dendrimers provide unique solutions to complex deliveryproblems for ocular drug delivery. Recent research efforts for improvingresidence time of pilocarpine in the eye was increased by using PAMAM dendrimerswith carboxylic or hydroxyl surface groups. These surface-modified dendrimers werepredicted to enhance pilocarpine bioavailability [57].
Dendrimers in pulmonary drug delivery
Dendrimers have been reported for pulmonary drug delivery of Enoxaparin. G2 and G3 generation positively charged PAMAM dendrimers increased the relative bioavailability of Enoxaparin by 40 % [58].
Dendrimer in transdermal drug delivery-
Dendrimers designed to be highly watersoluble and biocompatible have been shown to be able to improve drug properties such as solubility and plasma circulation time via transdermal formulations and to deliver drugs efficiently. PAMAM dendrimer complex with NSAIDs (e.g. Ketoprofen, Diflunisal) could be improving the drug permeation through the skin as penetration enhancers [59]. Ketoprofen and Diflunisal were conjugated with G5 PAMAM dendrimer and showed 3.4 and 3.2 times higher permeation. Chauhan et al. investigated enhanced bioavailability of PAMAM dendrimers by using indomethacin as the model drug in transdermal drug application. (chauhan) [60]
Dendrimer in oral drug delivery-
Oral drug delivery studies using the human colon adenocarcinoma cell line, Caco-2, have indicated that low-generation PAMAM dendrimers cross cell membranes, presumably through a combination of two processes, i.e. paracellular transport and adsorptive endocytosis. Remarkably, the Pgp efflux transporter does not appear to affect dendrimers, therefore drug dendrimer complexes are able to bypass the efflux transporter [63]. As increase in the concentration and generation, there was and methotrexate. PAMAM dendrimers conjugated with the folic acid and fluorescein isothiocyanate for targeting the tumor cells and imaging respectively. DNAassembled dendrimer conjugates may allow the combination of different drugs with different targeting and imaging agents so it is easy to develop combinatorial therapeutics [61].
Dendrimers for controlled release drug delivery
The anticancer drugs adriamycin and methotrexate were encapsulated into PAMAM dendrimers (i.e. G=3 and 4) which had been modified with PEG monomethyl ether chains (i.e. 550 and 2000 Da respectively) attached to their surfaces. A similar construct involving PEG chains and PAMAM dendrimers was used to deliver the anticancer drug 5-fluorouracil. Encapsulation of 5-fluorouracil into G=4 increase in the cytotoxicity and permeationof dendrimers.
Dendrimers in targeted drug delivery
Dendrimers have ideal properties which are useful in targeted drug-delivery system. One of the most effective cell-specific targeting agents delivered by dendrimers is folic acid PAMAM dendrimers modified with carboxymethyl PEG5000 surface chains revealed reasonable drug loading, a reduced release rate and reduced haemolytic toxicity compared with the non-PEGylated dendrimer. A third-generation dendritic unimolecular micelle with indomethacin entrapped as model drug gives slow and sustained in vitro release, as compared to cellulose membrane control [62].Controlled release of the Flurbiprofen could be achieved by formation of complex with amine terminated generation 4 (G4) PAMAM Dendrimers [63]. The results found that PEG-dendrimers conjugated with encapsulated drug and sustained release of methotrexate as compare to unencapsulated drug
Dendrimers in gene delivery
Dendrimer-based transfection agents have become routine tools for many molecular and cell biologist’s dendrimers are extensively used as non-viral vector for gene delivery. The use of dendrimers as gene transfection agents and drug-delivery devices have been extensively reviewed part [64]. Various polyatomic compound such as PEI, polylysine, and cationic have been utilized as non-viral gene carrier
Dendrimer as solubility enhancer
Dendrimers have hydrophilic exteriors and hydrophilic interiors, which are responsible for its unimolecular micellar nature. They form covalent as well as non-covalent complexes with drug molecules and hydrophobes, which are responsible for its solubilisation behavior [65].
Cellular delivery using dendrimer carrier
Dendrimer–ibuprofen complexes entered the cells rapidly compared with pure drug (1 hr versus>3 hr), suggesting that dendrimers can efficiently carry the complexes drug inside cells. PAMAM dendrimers were surfaceengineered with lauryl chains to reduce toxicity and enhance cellular uptake [66].
Therapeutic application
Dendrimers in photodynamic therapy
The photosensitizer 5-aminolevulinic acid has been attached to the surface of dendrimers and studied as an agent for PDT of tumorigenic keratinocytes [67]. This cancer treatment involves the administration of a light- activated photosensitizing drug that selectively concentrates in diseased tissue.
Dendrimers for boron neutron capture therapy
Boron neutron capture therapy (BNCT) refers to the radiation generated from the capture reaction of low-energy thermal neutrons by 10B atoms, which containapproximately 20% natural boron, to yield particles and recoiling lithium-7 nuclei. This radiation energy has been used successfully for the selective destruction of tissue. Dendrimers are a very fascinating compound for use as boron carriers due to their well defined structure and multivalency. The first example of a boroncontaining PAMAM dendrimer was synthesized by Barth et al [68]
Diagnostic application
Dendrimers as molecular probes
Dendrimers are fascinating molecules to use as molecular probes because of their distinct morphology and unique characteristics. For example, the immobilization of sensor units on the surface of dendrimers is a very efficient way to generate an integrated molecular probe, because of their large surface area and high density of surface functionalities [69]
Dendrimers as X-ray contrast agents
The X-ray machine is one of the fundamental diagnostic tools in medicine, and is applicable to numerous diseases. To obtain a high resolution X-ray image, several diseases or organs, such as arteriosclerotic vasculature, tumors, infarcts, kidneys or efferent urinary, require the use of an X-ray contrast agent. Dendrimers are currently under investigation as potential polymeric X-ray contrast agents. Krause and co-workers synthesized a number of potential dendritic X-ray contrast agents using various organo metallic complexes such as bismuth and tin [70,71]
Dendrimers as MRI contrast agents
A number of research groups have explored the use of dendrimers as a new class of high molecular weight MRI contrast agents. Wiener and coworkers developed a series of Gd(III)–DTPA-based PAMAM dendrimers [72]. To improve the pharmacokinetic properties of dendrimer contrast agents, introduction of target specific moieties to the dendritic MRI contrast agents have been considered. Wiener et al [73] synthesized a folate conjugated Gd(III)–DTPA PAMAM dendrimer, which increased the longitudinal relaxation rate of tumor cells expressing the high affinity folate receptor.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION:
The architecture of dendrimer, their shape, branching length and density, and their surface functionality, makes dendrimer ideal carriers for the various applications like drug delivery, therapeutic and diagnostic agent. Poor solubility, bioavailability , permeability biocompatibility and toxicity can be overcome by use of dendrimer.
REFERENCES:
1.Tomalia DA, and Frechet JMJ. Discovery of dendrimers and dendritic polymers: a brief historical perspective. J. Polym. Sci. A. 40: 2719-2728 (2002).
2 Buhleier E, Wehner W and Vogtle F: Synthesis of Molecular Cavity Topologies, Synthesis 1978; 2: 155-158.
3 Tomalia DA, Baker H, Dewald J, Hall M, Kallos G and Martin S: A New Class of Polymers: Starburst-Dendritic Macromolecules, Polymer. J 1985; 17(1): 117-132.
4. Lecuit M, Ohayon H, Braun L, Mengaud J and Cossart P: Stem cell Internal in of listeria monocytogenes with an intact leucine 2001; 369-377.
5. Newkome GR, Yao ZQ, Baker GR and Gupta VK: Cascade molecules: A new approach to micelles, a [27]-arborol. J. Org. Chem 1985; 50: 2003–2006.
6. Svenson S: Controlling surfactant self assembly. Curr. Opin. Colloid Interface Sci.2004; 9: 201–212.
7. Delie F, Berton M, Allemann E and Gurny R: Comparison of rich repeat region.1997: 5309 - 19.
8. Gilat SL, Adronov A and Frechet JJ light harvesting and energy transfer in novel convergently constructed dendrimers. Chem, Int. Edn. 1999; 38:1422-27
9. Caminati G, Turro NJ and Tomalia, DA: Photo physical investigation of starburst dendrimers and their interactions with anionic and cationic surfactants. J. Am. Chem. Soc. 1990; 112: 8515–8522.
10. Fischer M and Vögtle F: Dendrimers: From design to applications – A progress report. Angew. Chem, Int. Edn. 1999; 38: 884–905.
11 Tomalia DA, Naylor AM and Goddard WA: Starburst dendrimers: Molecularlevel control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem., Int. Edn. 1990; 29: 138–175.
12. Hawker CJ and Freshet, JMJ: Unusual macromolecular architectures: The convergent growth approach to dendritic polyesters and novel block copolymers. J. Am. Chem. Soc. 1992; 114: 8405–8413.
13. Zimmerman SC, Zeng F, Reichert D and Kolotuchin SV: Self-assembling dendrimers. Science 1996; 271: 1095–1098.
14. Chen, H.T., Neerman, M.F., E.,E.Simanek ., 2004. Cytotoxicity, hemolysis and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery, J. Am. Chem. Soc., 126, 10044-10048.
15. Jevprasesphant, R., Penny ,J., Jalal, R., Attwood, D., McKeown ,N.B., and Emanuele, D., 2003.The influence of surface modification on the cytotoxicity of PAMAM dendrimers, Int. J. Pharm., 252, 263-266.
16. El-Sayed ,M., Ginski, M., Rhodes ,C and Ghandehari ,H., 2002. Trans epithelial transport of poly (amidoamine) dendrimers across Caco-2 cell monolayers, J. Control.Release ., 81, 355–365
17.. FischeLi .Y., Ahlemeyer ,B., Krieglstein ,J., Kissel, T., 2003. In vitro cytotoxicitytesting of polycations: influence of polymer structure on cell viability and shemolysis, Biomaterials., 24, 1121–1131.
18. Malik ,N., Wiwattanapatapee, R., Klopsch ,R.,Lorenz, K., Frey, H., Weener ,J.W., Meijer, E.W., Paulus, W., and Duncan, D,. 2000. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of I-125-labelled poly (amidoamine) dendrimers in vivo, J. Control. Release., 65, 133-148.
19. Kobayashi, H.S., Kawamoto, T., Saga, N., Sato,A., Hiraga, T., Ishimori, J., K, Togashi and M.W. Brechbiel., 2001. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents, Magn. Reson. Med., 46, 781-788.
20. Patri AK, Majoros IJ, Baker JR, Dendritic polymer macromolecular carriers for drug delivery, Curr Opin Chem Biol.,6,2002, 466-471.
21. Morgenroth F, Reuther E, Mullen K, Polyphenylene Dendrimers: From Three-Dimensional to Two-Dimensional Structures Angewandte Chemie, International Edition in English,36 (6),1997, 631-634.
22. Nanjwade BK, Hiren M, Dendrimers: Emerging polymers for drug-delivery systems, Eur J Pharm Sci., 38 (3),2009,185-196.
23. Petar R, Dvornic L, Douglas S, Michael J, Owen SP, Radially Layered Copoly(amidoamin organosilicon) Dendrimers, United States Patent, 5,1998,739.
24. Dvornic PR, Owen MJ, Poly (amidoamine organosilicon) Dendrimers and Their Derivatives of Higher Degree of Structural Complexity, Synthesis and Properties of Silicones and Silicone-Modified Materials,2002,236-259.
25. Tomalia DA, Dewald JR, Hall MR, Martin SJ, Smith PB, Preprints 1st SPSJ Polym. Conf. Soc. Polym. Sci. Jpn, Kyoto, 1984, 65.
26. Hawker C, Fréchet JJ, J. Chem. Soc. Chem. Commun, 1990, 1010.
27. Brabander-van den Berg EMM, Meijer EW, Poly (propylene imine) Dendrimers: Large Scale Synthesis by Heterogenously Catalyzed Hydrogenation, Angew Chem Int Ed Engl, 32, 1308-1311.
28. Ritzén A, Frejd T, Synthesis of a chiral dendrimer based on polyfunctional amino acids, Chem. Commun, 1999, 207- 208.
29. Colinger M, Biological applications of dendrimers, Curr. Opin. Chem. Biol.,6,2002, 742–748.
30. Yiyun C, Zhenhua X, Minglu M, Tonguen X, Dendrimers as Drug Carriers:Applications in Different Routes of Drug, J.Pharma.Sci., 97(1),2008,123-143.
31. Hawker C, Wooley KL, Fréchet JMJ, J.Chem. Soc. Perkin, Trans,1,1993, 1287-1289
32. Sonke S and Tomalia DA: Dendrimers in biomedical applications reflections on the Field. Advanced Drug Delivery Reviews 2005; 57: 2106 – 2129.
33. Barbara K and Maria B: Review Dendrimers: properties and applications. Acta Biochimica Polonica 2001; 48: 199-208
34. Achar S, Puddephatt RJ, Organoplatinum dendrimers formed by oxidative addition, Angew. Chem., Int. Ed. Engl.,33,1994, 847–849.
35. Miller LL, Duan RG, Tully DC, Tomalia DA, Electrically conducting dendrimers, J. Am. Chem. Soc.,119, 1997, 1005–1010.
36. Wilken R, Adams J, End group dynamics of fluorescently labeled dendrimers, Macromol. Rapid Commun,18, 1997, 659– 665
37. Hummelen J.C, Van Dongen JLJ, Meijer EW, Electrospray mass spectrometry of poly(propylene imine) dendrimers— the issue of dendritic purity or polydispersity, Chem. Eur. J.,3, 1997, 1489– 1493.
38. Kallos GJ, Tomalia DA, Hedstrand DM, Lewis S, Zhou J, Molecular weight determination of a polyamidoamine starburst polymer by electrospray-ionization mass Spectrometry, Rapid Commun. Mass Spectrom,5, 1991, 383– 386.
39. Larre C, Bressolles D, Turrin C, Donnadieu B, Caminade AM, Majoral JP, Chemistry within mega molecules: regiospecific functionalization after construction of phosphorus dendrimers, J. Am. Chem. Soc.,120,1998, 13070– 13082.
40. Chu B, Hsiao BS, Small-angle X-ray scattering of polymers, Chem. Rev.,101,2001, 1727–1762.
41. Prosa TJ, Bauer BJ, Amis EJ, Tomalia DA, Scherrenberg R, A SAXS study of the internal structure of dendritic polymer systems, J. Polym. Sci., Part B, Polym. Phys.,35,1997,2913– 2924.
42. Rietveld IB, Smit JAM, Colligative and viscosity properties of poly(propylene imine) dendrimers in methanol, Macromolecules,32,1999, 4608– 4614
43. Topp A, Bauer BJ, Klimash JW, Spindler R, Tomalia DA, Amis EJ, Probing the location of the terminal groups of dendrimers in dilute solution, Macromolecules,32,1999,7226–7231.
44. Hofkens J, Verheijen W, Shukla R, Dehaen W, De Schryver FC, Detection of a single dendrimer macromolecule with a fluorescent dihydropyrrolopyrroledione (DPP) core embedded in a thin polystyrene polymer film, Macromolecules,31,1998,4493– 4497.
45. Gensch T, Hofkens J, Heirmann A, Tsuda K, Verheijen W, Vosch T, et al, Fluorescence detection from single dendrimers with multiple chromophores, Angew. Chem., Int. Ed. Engl.,38,1999, 3752–3756.
46. Zeng F, Zimmerman SC, Kolotuchin SV, Reichert DEC, Ma Y, Supramolecular polymer chemistry: design, synthesis, characterization, and kinetics, thermodynamics, and fidelity of formation of self-assembled dendrimers, Tetrahedron,58,2002, 825– 843.
47. Francese G, Dunand FA, Loosli C, Merbach AE, Decurtins S, Functionalization of PAMAM dendrimers with nitronyl nitroxide radicals as models for the outer-sphere relaxation in dendritic potential MRI contrast agents, Magn. Reson. Chem.,41,2003,81– 83.
48. Tabakovic I, Miller LL, Duan RG, Tully DC, Tomalia DA, Dendrimers peripherally modified with anion radicals that form C-dimers and C-stacks, Chem. Mater,9,1997,736–745
49. Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR, Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers, Proc. Natl. Acad. Sci. U. S. A.,93,1996, 4897– 4902.
50. Mourey TH, Turner SR, Rubinstein M, Fre´chet JMJ, Hawker CJ, Wooley KL, Unique behavior of dendritic macromolecules: intrinsic viscosity of polyether dendrimers,Macromolecules,25,1992, 2401– 2406.
51. Matos MS, Hofkens J, Verheijen W, De Schryver FC, Hecht S, Pollak KW. et al., Effect of core structure on photophysical and hydrodynamic properties of porphyrin dendrimers, Macromolecules,33,2000, 2967–2973.
52. Dantras E, Dandurand J, Lacabanne C, Caminade AM, Majoral JP, Enthalpy relaxation in phosphorus-containing dendrimers, Macromolecules,35, 2002,2090– 2094.
53. Trahasch B, Stu B, Frey H, Lorenz K, Dielectric relaxation in carbosilane dendrimers with perfluorinated end groups, Macromolecules,32, 1999, 1962–1966.
54. Pavlov GM, Errington N, Harding SE, Korneeva EV, Roy R, Dilute solution properties of lactosylated polyamidoamine dendrimers and their structural characteristic, Polymer,42, 2001,3671– 3678.
55. Wooley KL, Hawker CJ, Frechet JMJ, Unsymmetrical threedimensional macromolecules: preparation and characterization of strongly dipolar dendritic macromolecules, J. Am. Chem. Soc.,115,1993, 11496– 11505.
56. Zhuo RX, Du B, Lu ZR, In vitro release of 5-fluorouracil with cyclic core dendritic polymer, J. Control. Release,57, 249– 257.
57. Tolia,G.T ., Choi, H.H and Ahsan, F., 2008.The role of dendrimers in drug delivery, Pharmaceut Tech., 32, 88–98.
58. Dendrimers as a carrier for pulmonary delivery of enoxaparin, a low molecular weight heparin, J. Pharm. Sci. 2007. 96, 2090–2106.
59. Cheng, N. Man., T. Xu., R. Fu., X. Wang., X. Wang and L. Wen., 2007 .Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers, J. Pharm. Sci., 96, 595–602.
60 Chauhan, A.S., Sridevi,S., Chalasani, K.B., Jain, A.K., Jain, S.K., Jain, N.K and. Diwan, P.V., 2003 Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin, J. Control. Release. 90, 335-343.
61. Emanuele, D., Jevprasesphant ,A. R., Penny, R. J. and Attwood, D., 2004. J. Controlled Release. 95, 447-453
62. Choi ,Y., Thomas, T., Kotlyar ,A and Baker ,J.R., 2005 . Synthesis and functional evaluation of DNA-assembled polyamidoamine dendrimer clusters for cancer cell-specific targeting, Chem. Biol., 12, 35–43.
63. Patri, A.K., Majoros and. Baker, J.R., 2002. Dendritic polymer macromolecular carriers for drug delivery, Curr. Opin. Chem. Biol., 6, 466-471.
64. Asthana ,A., Chauhan, A.S., Diwan, P.V and. Jain ,N.K., 2005 . Poly (amidoamine) (pamam) dendritic nanostructures for controlled site specific delivery of acidic anti-inflammatory active ingredient, AAPS PharmSciTech. 6, Article 67. 269
65. Broeren, M.A.C., van ,J.L.J., Dongen,M., Pittelkow, J.B., Christensen, M.H.P., Genderen .v ., 2004.Multivalency in the gas phase: the study of dendritic aggregates by mass spectrometry, Angew. Chem., Int. Ed. Engl., 43, 3557–3562.
66. Mohammad N and Antony D., 2006. Crossing cellular barriers using dendrimer nanotechnologies, Current Opinion in Pharmacology, 6, 522–527.
67. Sonke S and Tomalia D.A., 2005. Dendrimers in biomedical applications reflections on the Field, Advanced Drug Delivery Reviews., 57, 2106 – 2129.
68. Barth, R.F., Adams, D.M., Soloway, A.H., Alam. F and Darby, M.V., 1994. Boronated starburstdendrimer-monoclonal antibody immunoconjugates., 5, 58–66.
69. Albrecht, M., Gossage ,R.A., Lutz ,M., Spek ,A.L and Van Koten G., 2000. Diagnostic organometallic and metallodendritic materials for SO2 gas detection: reversible binding of sulfur dioxide to arylplatinum(II) complexes. Chem Eur J., 6, 1431- 1445.
70. Schumann, H., Wassermann, B.C., Schutte, S., Velder, J., Aksu, Y and Krause, W., 2003 .Synthesis and characterization of water-soluble tin-based metallodendrimers. Organometallics; 22, 2034-41.
71 Krause, W., Hackmann-Schlichter N., Maier, F.K., Muller, R., 2000. Dendrimers in diagnostics. Topics Curr Chem., 210, 261–308.
72. Wiener, E.C., Brechbiel, M.W, Brothers, H., Magin, R.L., Gansow, O.A and Tomalia, D.A., 1994. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med; 31, 1-8.
73 Wiener, E.C., Konda, S., Shadron ,A., Brechbiel , M and Gansow,O., 1997.Targeting dendrimer– chelates to tumors and tumor cells expressing the highaffinity folate receptor. Invest Radiol; 32: 748-54
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









