{ DOWNLOAD AS PDF }
About Authors:
1Avinash M Nagapara*, 2Hasmukh C Nagapara, 1Darshan Madiya, 1Shital Faldu
1Department of Quality Assurance, Smt. R. D. Gardi B. Pharmacy College, Rajkot, Gujarat
2Virani Science College, Rajkot, Gujarat
*avinashnagapara@yahoo.com
Abstract
A stress Degradation study of the combination containing Ciprofloxacin Hydrochloride and Bromhexine Hydrochloride was carried out by various reagent like 1 M NaOH, 1M HCl, 6% H2O2 and Neutral water at higher temperature. This combination is widely used for the treatment of various types of respiratory disorders and COPD. The effects of the various stress conditions were observed in terms of decrease in the peak hight, increase in peaks or slightly change or shifting of the wavelengths. It was found that by applying various stress conditions, 4% to 50% drugs were degraded in case of pure drugs as well as the Commercial formulation. Thus spectrophotometry was successfully utilised for primary stability study of the pure drug as well as the degradation study of Ciprofloxacin Hydrochloride and Bromhexine Hydrochloride.
INTRODUCTION:
Ciprofloxacin HCl (fig. 1a) is the salt of Ciprofloxacin HCl, chemically known as, 1-cyc1opropyl-6-fluoro-1, 4- dihydro-4-oxo-7(1 piperazinyl)-3-quinolinecarboxylic acid hydrochloride monohydrate[1]. it is a first generation fluoroquinolone.Its spectrum of activity includes most strains of bacterial pathogens responsible for respiratory, urinary tract, gastrointestinal, and abdominal infections, including Gram-negative and gram- positive bacterial pathogens[2]. Bromhexineis a synthetic derivative of the herbal active ingredient vasicin, they chemically known as 2-amino-3,5-dibromobenzyl(cyclohexyl)methylamine hydrochloride[1]. CIPRO is official in USP, BP, EP & IP whereas BROM is official in USP, BP, EP. The chemical structures of CIPRO & BROM are shown in Fig. 1.Combination drug products of CIPRO and BROM are widely marketed and used in the treatment of mucous plugs, patient with Chronic obstructive lung disease and bronchitis.
Some procedures have been reported for the degradation study of CIPRO and BROM in single dosage forms . But for the combination no one method is found. From the literature, no degradation studies could be founded for BROM and CIPRO combination. To check the degradation of the drugs by applying various stress conditions as well as the stability study of various drugs is one of important criteria for an analyst [2-4]. Therefore degradation study of this combination in combined dosage forms seemed to be necessary. An experiment was made to find the degradation of these drugs individually and with their combination using various stress conditions like refluxing the drugs with high temperature using highly acidic, alkaline or neutral conditions.
Fig. 1 Chemical structures of the analytes (1a) CIPRO & (1b) BROM
Materials and Methods
Site of experimentation: Smt. R. D. Gardi B. Pharmacy College, Nyara, Rajkot
1. INSTRUMENTATION: UV visible spectrophotometer Helios Alpha, Thermo Scientific, (model UV A 1002E) with 1 cm matched quartz cells were used for all absorbance measurements. Contech, EIE instrument pvt. Ltd. (model CA 34) balance was used for weighing the samples
2. REAGENTS: Double distilled water and Whatmann filter paper (0.45μm) were used for filtration. Active pharmaceutical ingredient (API) of Ciprofloxacin HCl (CIPRO), Bromhexine HCl (BROM) were obtained as gift sample from Aarti drugs, Mumbai and Ven petrochem & pharma PVT. LTD. (tablets with composition BROM-8 mg and CIPRO-250 mg) were procured from the local market.
3. CHEMICAL: Methanol, NaOH, Hydrogen Peroxide and HCl are obtained from local market.
4. SOLUTION:
Stock solutions, 1 mg mL-1 of pure samples of CIPRO and BH were freshly prepared individually in methanol. For acidic degradation, 1M HCl solutions were prepared. To study neutral degradation, doubled distilled water was used for preparing various solutions. To study alkaline degradation, 1M NaOH solutions were prepared. For oxidative degradation, 6% H2O2 were used.
PROCEDURE:
1ml of stock solution of pure CIPRO, (1 mg mL-1), was taken to a round bottom flask containing 9ml of 1M HCl. After ten times dilution, the absorbance of resultant solution was taken using blank solution that contained all except the drug and was named as zero time reading. Then solution was reflux at 60°C for 120 minutes on an oil bath.
During refluxing process, the samples were taken after 30 minutes, 60 minutes and 120 minutes intervals. Then the resultant solutions were cooled and absorbance of the resultant solutions were measured using blank treated by the same way after ten time dilutions of each. The same procedure was applied for different degradation conditions like refluxing with neutral condition using distilled water, 1M NaOH, 6% H2O2. The overall procedure was followed for pure BH, pure CIPRO and commercial formulation of both the drugs. The solution of commercial formulation was prepared in 1: 1 ratio by standard addition method. The table 1 shows the sampling plan total degradation study.
Tables 1 Sampling plan for estimation of degraded products [5-9].
|
Type of Degradation |
Solution used for degradation study |
Sampling plan |
|
Acidic degradation |
Reflux at 60°C with 1M HCl |
Initial, 60 minutes, 120 minutes |
|
Neutral degradation |
Distilled water, reflux at 60°C |
Initial, 60 minutes, 120 minutes |
|
Alkaline degradation |
Reflux at 60°C with 1M NaOH |
Initial, 60 minutes, 120 minutes |
|
Oxidative degradation |
6% H2O2 and reflux at 80°C |
Initial, 60 minutes, 120 minutes |
RESULT AND DISCUSSION:
Table 2 The results of the acidic degradation studies were observed in the table given below:
|
Drug |
Condition |
Amt. of drug degraded |
|
Ciprofloxacin HCl |
After refluxing with 1M HCl at 60°C for- Initial 60 minute and 120 minute
|
25.98 % ciprofloxacin hydrochloride is degraded after 120 minute refluxing. |
|
Bromhexine HCl |
After refluxing with 1M HCl at 60°C for- Initial 60 minute and 120 minute
|
31.73 % bromhexine hydrochloride is degraded after 120 minute refluxing. |
|
Combination (Cinor-BR) |
After refluxing with 1M HCl at 60°C for- Initial 60 minute and 120 minute |
In combination 12.05 % and 13.35 % degradation of ciprofloxacin hydrochloride and bromhexine hydrochloride respectively. |
By applying highly acidic conditions,
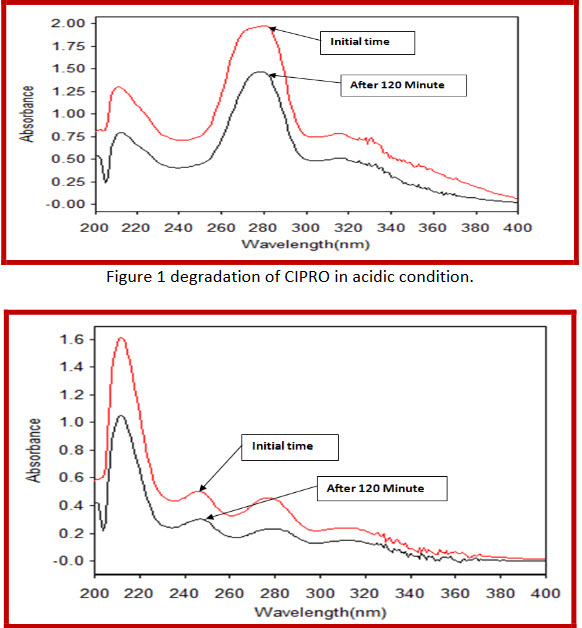
Figure 2 degradation of BROM in acidic condition.
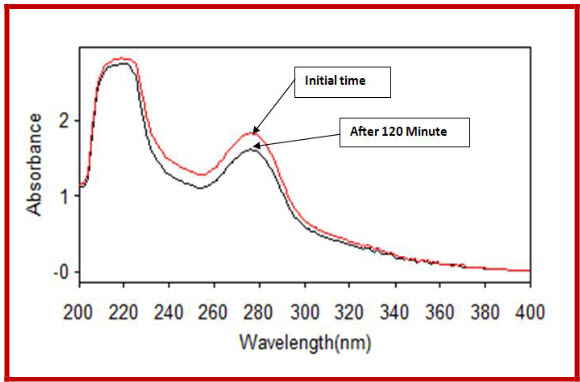
Figure 3 Degradation of CIPRO and BROM in acidic condition.
Table 3 The results of the neutral degradation studies were observed in the table given below:
|
Drug |
Condition |
Amt. of drug degraded |
|
Ciprofloxacin HCl |
After refluxing with Distilled water at 60°C for- Initial 60 minute and 120 minute |
4.31 % ciprofloxacin hydrochloride is degraded after 120 minute refluxing. |
|
Bromhexine HCl |
After refluxing with Distilled water at 60°C for- Initial 60 minute and 120 minute |
8.75 % bromhexine hydrochloride is degraded after 120 minute refluxing. |
|
Combination (Cinor-BR) |
After refluxing with Distilled water at 60°C for- Initial 60 minute and 120 minute
|
In combination 13.57 % and 4.31 % Degradation of ciprofloxacin hydrochloride and bromhexine hydrochloride respectively. |
By applying Neutral conditions,
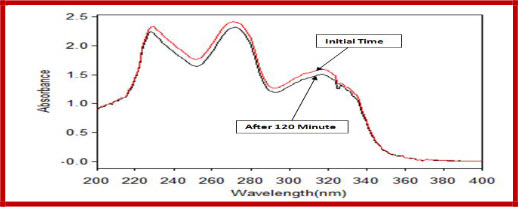
Figure 4 Degradation of CIPRO in neutral condition
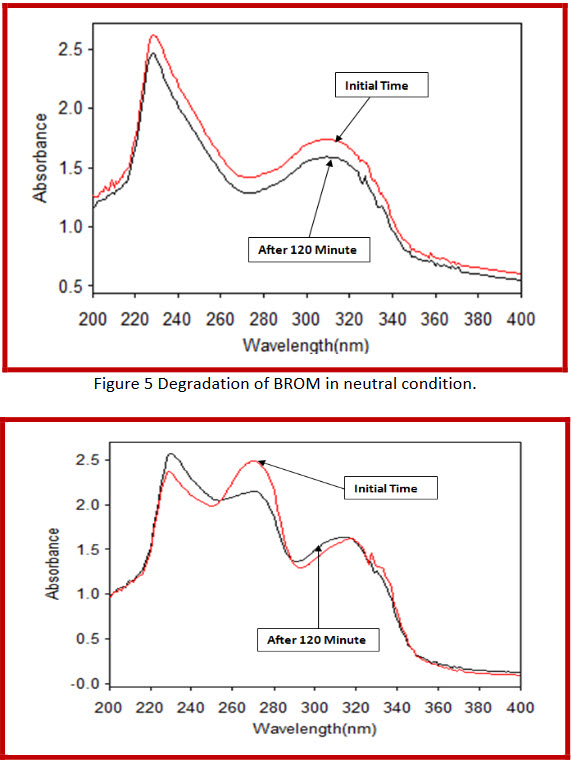
Figure 6 Degradation of CIPRO and BROM in neutral condition
Table 4 The results of the basic degradation studies were observed in the table given below:
|
Drug |
Condition |
Amt. of drug degraded |
|
Ciprofloxacin HCl |
After refluxing with 1M NaOH at 60°C for- Initial 60 minute and 120 minute |
47.3 % ciprofloxacin hydrochloride is degraded after 120 minute refluxing. |
|
Bromhexine HCl |
After refluxing with 1M NaOH at 60°C for- Initial 60 minute and 120 minute |
52.56 % bromhexine hydrochloride is degraded after 120 minute refluxing. |
|
Combination (Cinor-BR) |
After refluxing with 1M NaOH at 60°C for- Initial 60 minute and 120 minute
|
In combination 45.78 % and 52.91 % degradation of ciprofloxacin hydrochloride and bromhexine hydrochloride respectively. |
By applying Basic conditions,
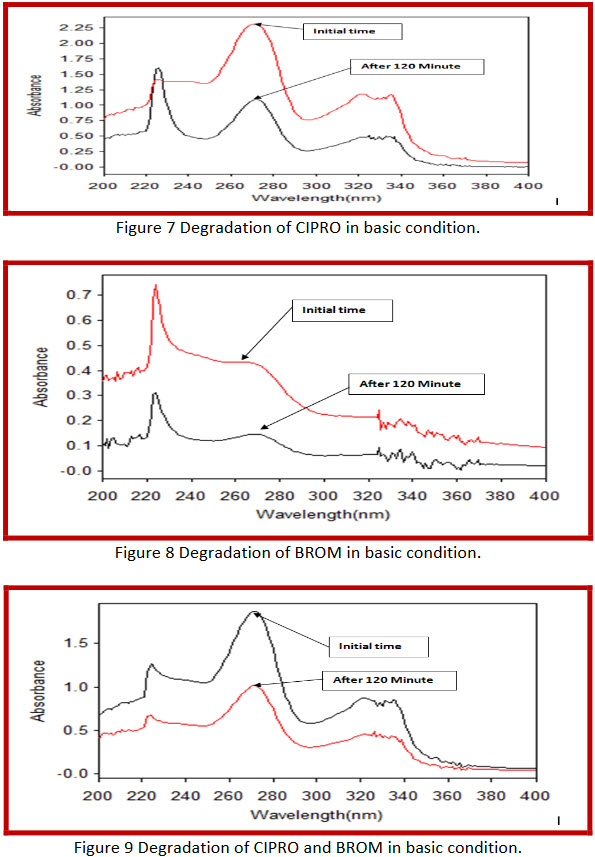
Table 5 The results of the oxidative degradation studies were observed in the table given below:
|
Drug |
Condition |
Amt. of drug degraded |
|
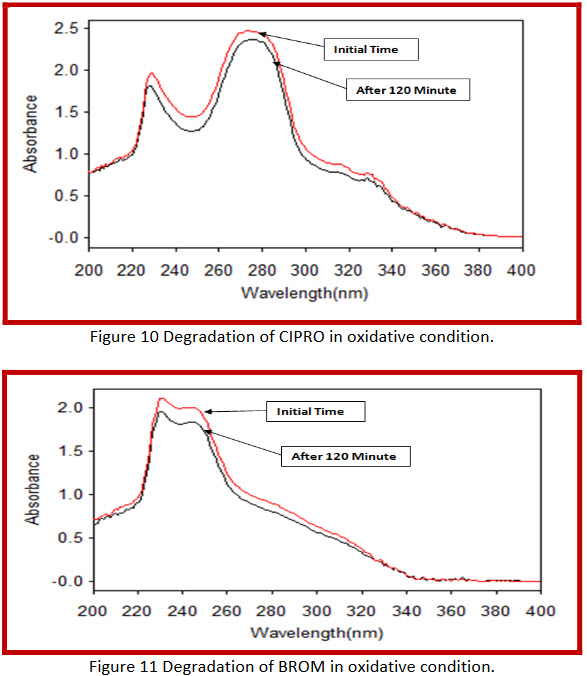
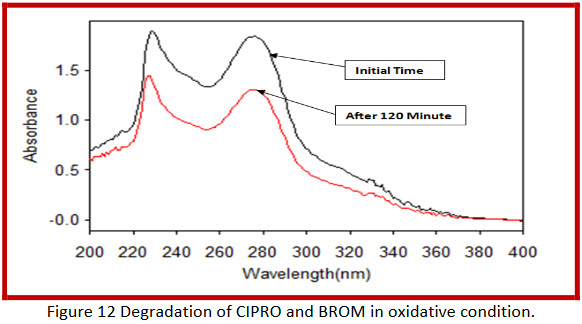
Ciprofloxacin HCl |
After refluxing with 6 % H2O2 at 60°C for- Initial 60 minute and 120 minute |
8.88 % ciprofloxacin hydrochloride is degraded after 120 minute refluxing. |
|
Bromhexine HCl |
After refluxing with 6 % H2O2 at 60°C for- Initial 60 minute and 120 minute |
4.58 % bromhexine hydrochloride is degraded after 120 minute refluxing. |
|
Combination (Cinor-BR) |
After refluxing with 6 % H2O2 at 60°C for- Initial 60 minute and 120 minute
|
In combination 29.64 % and 32.48 % degradation of ciprofloxacin hydrochloride and bromhexine hydrochloride respectively. |
By applying Oxydative conditions,
CONCLUSION
From the above experiment it was found that degradation was observed in CIPRO, BROM and Combined dosage form. In the study we saw the degradation in the form of decrease in peak height or increase in peak height or change in peak shape. Thus using spectrophotometry, we can get a better result of the degradation of the above combination.
REFERENCES
1. Indian Phamacopoeia, Volume-I & Volume-II, Government of India, Ministry of Health and Family Welfare, Published by The Indian Pharmacopoeia Commission, Ghaziabad, 2007, pp 1092,1093.
2. Rang HP, Dale MM, Ritter JM, Pharmacology, 4th Edn, Churchill Livingston, New York, 1999, pp 282, 344.
3. The United States Pharmacopoeia-30, NF-25, Asian edition, Rockville, MD: The US Pharmacopoeial Convention, Inc., 2004, pp 1756.
4. Tripathi KD, Essentials of Medical Pharmacology; 5thEdn; Jaypee brothers medical publishers, New Delhi, 2004, pp , 619,644.
5. Laine LM, Kaarina KPP and Tammilehto S. “Decomposition of salbutamol in aqueous solutions.The effect of buffer species, pH, buffer concentration and antioxidants.” International Journal of Pharmaceutics, 1995, 18, 189-195.
6. Nour TA, Yousry M and Howayda MA. “Potentiometric Flow Injection Analysis of Bromhexine Hydrochloride and its Pharmaceutical Preparation Using Conventional and Coated Wire Ion- Selective Electrodes.” Scientia Pharmaceutical, 2006, 74, 121-135.
7. Zaid AN. “Formulation and stability evaluation of 1% w/v oral solution of Bromhexine hydrochloride for veterinary use.” The Islamic University Journal, 2007, 15, 13 -22.
8. Kasawar GB, Farooqui M, “Development and validation of a stability indicating RPHPLC method for the simultaneous determination of related substances of albuterol sulfate and ipratropium bromide in nasal solution.” Journal of Pharmaceutical and Biomedical Analysis, 2010, 52, 19-29.
9. Hiral ND, Rajeshree CM, “Degradation study of salbutamol sulphate, bromhexine hydrochloride and etofylline in pure and in their ternary mixture by spectrophotometry.”International journal of pharmaceutical and health science. 2010, 1, 136-144.
REFERENCE ID: PHARMATUTOR-ART-2197
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 7 Received On: 26/04/2014; Accepted On: 04/05/2014; Published On: 01/07/2014How to cite this article: AM Nagapara, HC Nagapara, D Madiya, S Faldu; Degradation Study of Ciprofloxacin Hydrochloride, Bromhexine hydrochloride and their Combined Pharmaceutical Dosage Form by Spectrophotometry; PharmaTutor; 2014; 2(7); 102-109 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









