 About Authors:
About Authors:
C.P.Meher*, D.V.Charan, S.P.Sethy
Asst. Professor
Maheshwara Institute of Pharmacy,
Chitkul, Patancheru, Medak, A.P
*chaitanyameher84@gmail.com
ABSTRACT
Heterocyclic chemistry is the branch of chemistry dealing with structure,synthesis, properties, and applications of heterocycles. heterocyclic compounds may be inorganic, most contain at least one carbon. Since in organic chemistry non-carbons usually are considered to replace carbon atoms, they are called heteroatoms, meaning 'different from carbon and hydrogen' (rings of heteroatoms of the same element are homocyclic). The IUPAC recommends the Hantzsch-Widman nomenclature for naming heterocyclic compounds.The present review article is based on the condenced heterocyclic system quinoline derivatives with their tremendous pharmacological activities.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1558
INTRODUCTION
Quinoline is the condenced heterocyclic system.They are also termed as benzopyridines because it having fused benzene and pyridine rings. they are aromatic compounds. Quinoline is monoacidic tertiary bases and failed to react with nitrous acid but consume only one mole of alkyl halide to yield quaternary, ammonium salts. Quinolineis a heterocyclicaromatic organic compoundwith the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few application, but many of its derivatives are useful in diverse applications. A prominent example is quinine, which is found naturally in plants as alkaloids. 4-Hydroxy-2-alkylquinolines(HAQs) are involved in antibiotic resistance.Quinoline derivatives represent the major class of heterocycles, and a number of preparations have been known since the late 1800s. The quinoline ring system occurs in various natural products, especially in alkaloids. The quinoline skeleton is often used for the design of many synthetic compounds with diverse pharmacological properties. In 1820, quinine was isolated as the active ingredient from the bark of Cinchona trees and successively replaced the crude bark for the treatment of malaria. Despite its relatively low efficacy and tolerability, quinine still plays an important role in the treatment of multiresistant malaria. This molecule has also played a historical role in organic chemistry as a target for structural determination and total synthesis , and recently both stereoselective and enantioselective total syntheses.
The quinoline ring system is found in medicinal plant alkaloids and constitutes a key structural component of pharmaceuticals, agrochemical, dyestuffs, and materials. Because of its unique pharmaceutical importance, a continuous development in the synthesis of new quinoline derivatives is a growing area research. An impressively diverse range of efficient quinoline synthesis based on new metal-catalyzed coupling cyclizations or acid catalyzed cycloaddition of appropriate precursors have been developed during past years that proves practical importance of this heterocyclic system. However, having a plethora of new synthetic methods for preparing quinoline derivatives including classical syntheses, a clean, efficient, large-scale and cheap technology is still needed to obtain useful polyfuntionalizated quinolines . some of the important important properties of quinoline is given below in table-1
[adsense:468x15:2204050025]
Table-1
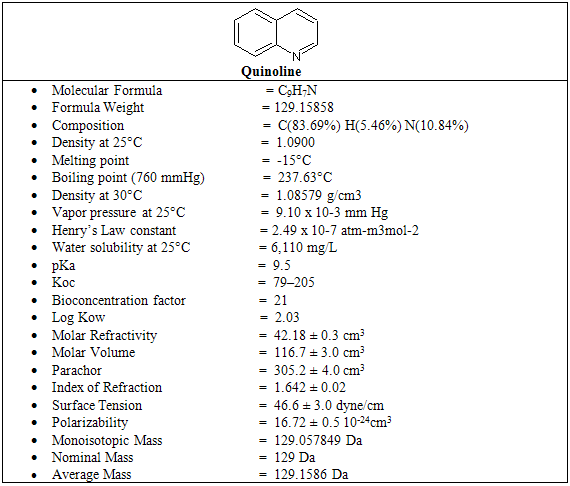
Sources of quinoline include petroleum, coal processing, wood preservation, production and use facilities, and shale oil . It is used as an intermediate in the production of various compounds including 8-hydroxyquinoline, hydroxyquinoline sulfate, and copper-8- hydroxyquinolate. Quinoline is also a solvent for resins and terpenes, and is used in the production of paints. Because of its strong absorption of light wavelengths >290 nm, quinoline has the potential for direct photolysis in the atmosphere. Removal from the atmosphere can occur via wet and dry deposition.
Postulated metabolic pathway for detoxification and activation of quinoline
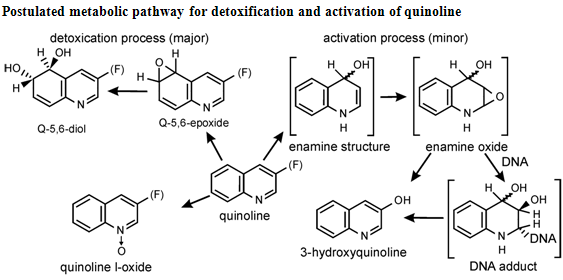
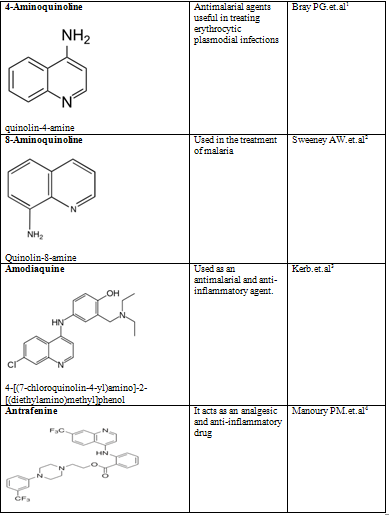
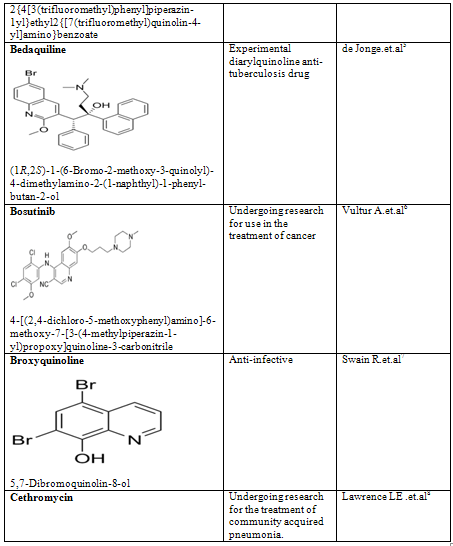
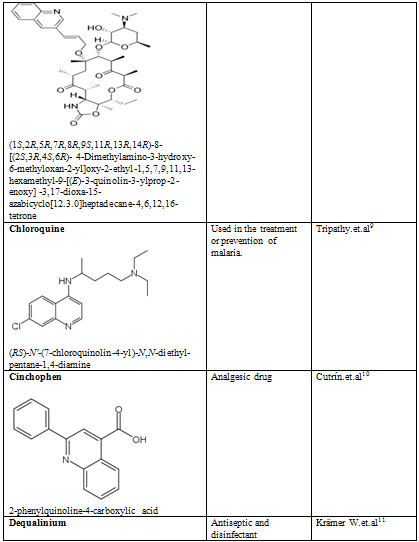
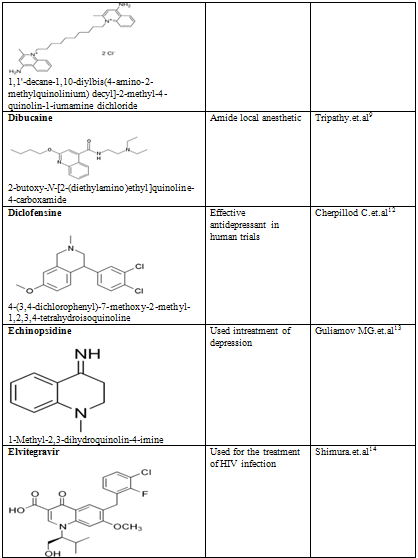
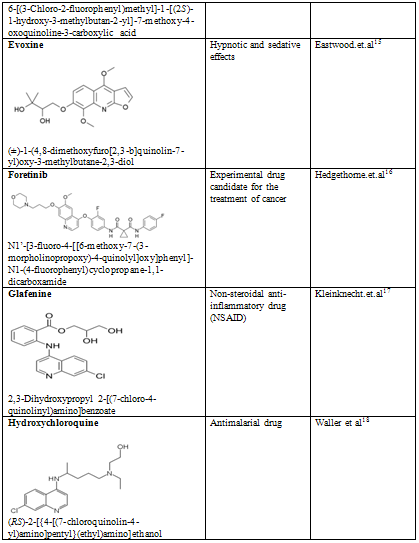
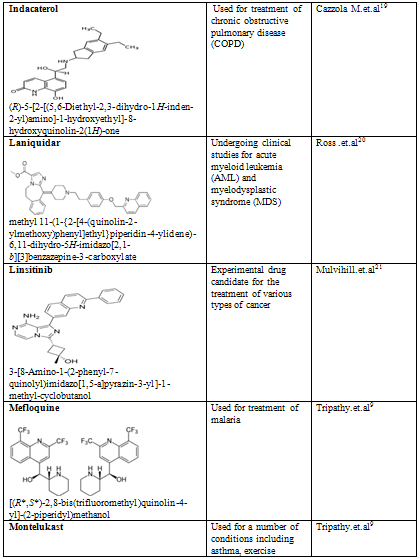
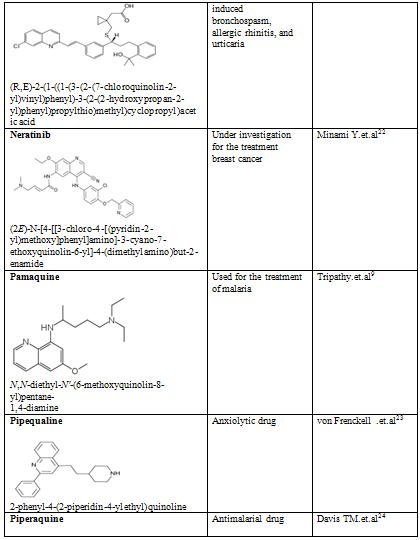
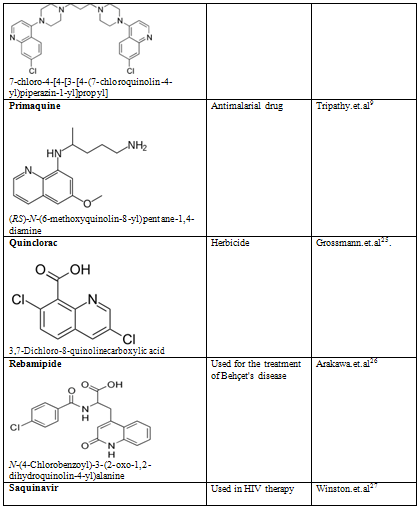
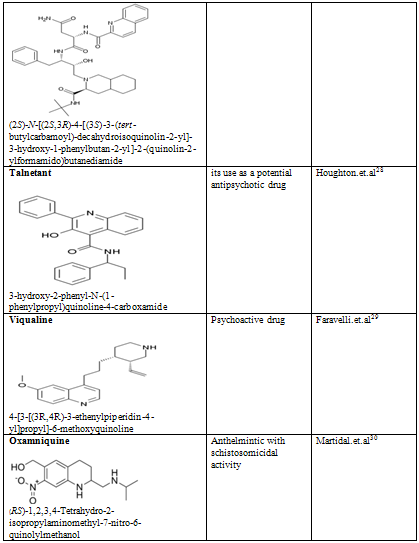
Quinoline shows a wide spectrum of biological activity. Alteration in the qunoline has numerous biological activity. Some of the marketed drugs & drugs under investigation are listed on on table-2.
Table-2
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
The reviewed quinoline moiety has shown a wide spectrum of biological activities. The various substituted quinoline are having significant antipsychotic activity, Significant analgesic, anti-inflammatory, antipyretic and anti tumor activity, is displayed by some effective substituted quinoline derivative which presently leading drug in the market in entire. some modified quinoline are found to to be effective as anti malarial, anxiolytic whereas some of the derivatives of quinoline are under investigation for the treatment of cancer, chronic obstructive pulmonary disease .some of the important marketed quinoline nucleus containing drug having different biological or pharmacological activity were discussed in table. The quinoline nucleus based pharmaceutical are rapidly becoming very important class of therapeutic agents and are likely to replace many existing organic based pharmaceuticals in the very near future. The quinoline based pharmaceuticals will be produced on a large scale by modern drug discovery company by different research development processes and will become available commercially for therapeutic use. With the key benefits including favorable time to market and high rate of success in clinical trial compared with traditional pharmaceuticals due to diverse biological action with less toxicity, so in future therapeutic quinoline drug will play a vital role in the treatment of different diseases. The biological profiles of this new generation of quinoline represent much progress with regard to the older compounds.
REFERENCES
1. Bray PG, Hawley SR, Ward SA (1996). "4-Aminoquinoline resistance of Plasmodium falciparum: insights from the study of amodiaquine uptake". Mol. Pharmacol. 50 (6): 1551–8.
2. Sweeney AW, Blackburn CRB, KH Rieckmann. (1 August 2004). "Short report: The activity of pamaquine, an 8-aminoquinoline drug, against sporozoite-induced infections of Plasmodium vivax (New Guinea strains)". Am J Trop Med Hyg 71 (2): 187–189.
3. Kerb, Reinhold; Fux, Morike, Kremsner, Gil, Gleiter (2009). "Pharmacogenetics of antimalarial drugs: effect on metabolism and transport". Lancet Infectious Disease 9: 760-774.
4. Manoury PM, Dumas AP, Najer H, Branceni D, Prouteau M, Lefevre-Borg FM. Synthesis and analgesic activities of some (4-substituted phenyl-1-piperazinyl)alkyl 2-aminobenzoates and 2-aminonicotinates. Journal of Medicinal Chemistry. 1979 May;22(5):554-9.
5. de Jonge MR, Koymans LH, Guillemont JE, Koul A, Andries K (June 2007). "A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910". Proteins 67 (4): 971–80.
6. Vultur A, Buettner R, Kowolik C, et al. (May 2008). "SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells". Mol. Cancer Ther. 7 (5): 1185–94
7. Swain R, Bapna JS (January 1986). "Impairment of exercise tolerance due to broxyquinoline-brobenzoxaldine combination". Hum Toxicol 5 (1): 63–4
8. Lawrence LE (June 2001). "ABT-773 (Abbott Laboratories)". Current Opinion in Investigational Drugs 2 (6): 766–72
9. Essential of pharmacology,K.D.Tripathy,6th edition,p-222,223,351,781
10. Cutrín Prieto C, Nieto Pol E, Batalla Eiras A, Casal Iglesias L, Pérez Becerra E, Lorenzo Zúñiga V (1991). "[Toxic hepatitis from cinchophen: report of 3 cases]" (in Spanish; Castilian). Medicina clínica 97 (3): 104–6
11. Krämer W. (1977). "[Treatment of tonsilitis with dequalinium choride]" (in German). Fortschr Med. 95 (16): 1108–10
12. Cherpillod C, Omer LM. A controlled trial with diclofensine, a new psychoactive drug, in the treatment of depression. Journal of International Medical Research. 1981;9(5):324-9
13. Guliamov MG (1982). "[Experience with the use of new Bulgarian psychotropic drugs]" (in Russian). Zhurnal Nevropatologii I Psikhiatrii Imeni S.S. Korsakova (Moscow, Russia : 1952) 82 (11): 115–22.
14. Shimura K, Kodama E, Sakagami Y, et al. (2007). "Broad Anti-Retroviral Activity and Resistance Profile of a Novel Human Immunodeficiency Virus Integrase Inhibitor, Elvitegravir (JTK-303/GS-9137)". J Virol 82 (2): 764
15. Eastwood, FW; Hughes, GK; Ritchie, E. (1954). "Alkaloids of the Australian Rutaceae: Evodia xanthoxyloides F.Muell. IV. The structures of Evoxine and Evoxoidine". Australian Journal of Chemistry 7 (1): 87–98
16. Hedgethorne, K., Huang, P.H. (2010). "Foretinib. c-Met and VEGFR-2 inhibitor, Oncolytic". Drugs Fut 35 (11): 893–901
17.Kleinknecht, D; Landais, P; Goldfarb, B (1986). "Analgesic and non-steroidal anti-inflammatory drug-associated acute renal failure: A prospective collaborative study". Clinical nephrology 25 (6): 275–81
18. Waller et al. 2nd ed.'medical pharmacology and therapeutics'pg 370
19. Cazzola M, Matera MG, Lötvall J (July 2005). "Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease". Expert Opin Investig Drugs 14 (7): 775–83.
20. Ross DD (2004). "Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome". Best practice & research. Clinical haematology 17 (4): 641–51
21. Mulvihill, MJ; Cooke, A; Rosenfeld-Franklin, M; Buck, E; Foreman, K; Landfair, D; O'Connor, M; Pirritt, C et al. (2009). "Discovery of OSI-906: A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor". Future medicinal chemistry 1 (6): 1153–71.
22. Minami Y, Shimamura T, Shah K, et al. (July 2007). "The major lung cancer-derived mutants of ERBB2 are oncogenic and are associated with sensitivity to the irreversible EGFR/ERBB2 inhibitor HKI-272". Oncogene 26 (34): 5023–7
23. von Frenckell R, Ansseau M, Bonnet D. Evaluation of the sedative properties of PK 8165 (pipequaline), a benzodiazepine partial agonist, in normal subjects. International Clinical Psychopharmacology. 1986 Jan;1(1):24-35.
24. Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF (2005). "Piperaquine: a resurgent antimalarial drug". Drugs 65 (1): 75–87.
25. Grossmann, K (1998). "Quinclorac belongs to a new class of highly selective auxin herbicides". Weed Science 46: 707–716.
26. Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K (1995). "Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine". Dig Dis Sci 40 (11): 2469–72.
27. Winston A, Back D, Fletcher C et al. (2006). "Effect of omeprazole on the pharmacokinetics of saquinavir-500 mg formulation with ritonavir in healthy male and female volunteers". AIDS 20 (10): 1401–6
28. Houghton LA, Cremonini F, Camilleri M, Busciglio I, Fell C, Cox V, Alpers DH, Dewit OE, Dukes GE, Gray E, Lea R, Zinsmeister AR, Whorwell PJ. Effect of the NK(3) receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterology and Motility. 2007 Sep;19(9):732-43
29. Faravelli C, Albanesi G, Sessarego A (1988). "Viqualine in resistant depression: a double-blind, placebo-controlled trial". Neuropsychobiology 20 (2): 78–81
30. Martidale, The Extra Pharmacopoeia, 31st ed, p121.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









