ABOUT AUTHORS:
*Md. JahaSultana, P. Vijaya Sri, G.Alekhya, S.VenkateswaraRao, Chaitanya Prasad Meher
Department Of Pharmaceutical Analysis
Vijaya Institute of Pharmaceutical Sciences For Women
Enikepadu, Vijayawada-521108, Andhra Pradesh, India
sohnivya786@gmail.com
ABSTRACT:
Over a past decades, nuclear magnetic resonance (NMR) has progressed rapidly in improvement of experimental method and development of novel approaches. NMR is a research technique that exploits the magnetic properties of certain atomic nuclei. Of all the spectroscopy methods, NMR is the only one of which a complete analysis and interpretation of the entire spectrum is normally excepted. Although larger amounts of samples are needed when compared with mass spectroscopy, NMR is non-destructive and with modern instruments good data may be obtained from samples weighing less than a milligram. In this review a brief introduction to the NMR is given which includes instrumentation, parameters influencing NMR and applications.
REFERENCE ID: PHARMATUTOR-ART-2076
INTRODUCTION(1, 2, 6):
Over the past fifty years Nuclear Magnetic Resonance spectroscopy, commonly referred to as NMR, has become the dominant method of analysis of organic compounds, because in many cases it provides a way to determine an entire structure using one set of analytical tests. It is also increasingly used in inorganic chemistry and biochemistry, where it also provides a lot of valuable structural information. NMR is a property of the nucleus of an atom, concerned with what is known as nuclear spin (I). This is equivalent to the nucleus acting like a tiny bar magnet. Isotopes can have a variety of values for I (including zero). In this NMR spectroscopy only nucleus containing odd mass number (A) or odd atomic number (Z) are most useful which includes hydrogen 1 (1H), carbon 13 (13C), fluorine 19 (19F) and phosphorus 31 (31P). Whereas nucleus containing even mass number (A) or even atomic number (Z) are not used , This includes 10B, 14N etc.
Resonance frequency of a particle, substance is the key feature of NMR and is directly proportional to the strength of the applied magnetic field.The effectiveness of NMR can also be improved by using following techniques
- Hyperpolarization
- Two-dimensional
- Three-dimensional
- Higher-dimensional multi frequency techniques
Although large amounts of sample are needed when compared with mass spectroscopy, NMR isnon-destructive and with modern instruments good data may be obtained from samples weighing less than a milligram.The 1H nucleus is most commonly studied by using NMR spectroscopy because of its high natural abundance (99.98%) and the fact that it is invariably present in the majority of organic compounds. PMR provides information about the number of different types of protons and also regarding the nature of the immediate environment of each of them. Despite of NMR, Carbon- 13 is also an important nucleus because carbon forms the backbone of all organic compounds and valuable structural information can be derived by 13C NMR spectroscopy.
DEFINITIONS(2, 3, 6):
Spectroscopyis a method used to study the light as a function of length of the wave that has been emitted, reflected through solid, liquid or gas. Nuclear Magnetic Resonance (NMR) is a spectroscopy technique which is based on the transition of Electromagnetic radiations in a radio frequency region 4 to 900 MHz by nuclei of atoms in the presence of magnetic field. In this radio frequency radiations are used to induce transitions between different nuclear spin states of samples in a magnetic field. When proton (hydrogen) is studied then it is called as Proton Magnetic Resonance (PMR). When other nuclei like 13C, 9F, 35Cl etc. are studied then it is called as NMR.
HISTORY(2, 3, 5, 6):
NMR was first described and measured in molecular beams by Isidor Rabi in 1938 by extending the Stern-Gerlach experiment and in 1944; Rabi was awarded the Nobel Prize in physics for this work. In 1946, Felix Bloch and Edward Mills Purcell expanded the technique for use on liquids and solids, for which shared the Nobel Prize in physics 1952. Purcell had worked on the development of radar during World War II. His work during that project on the production and detection of radio frequency power and on the absorption of such radio frequency power by matter laid the foundation for Rabi’s discovery of NMR. In 1991 Richard Ernst awarded the Nobel Prize for his contributions to high resolution NMR and in 2002 Wuthrichawarded the Nobel Prize for his development of three dimensional structure of biological macromolecules in solution. In 2003 Lauterbur and Mansfield awarded the Nobel prize for their discoveries regarding magnetic resonance imaging (MRI). In the past decades, NMR spectroscopy has achieved a great development in multinuclear studies, very high field operation comes to being, applications of new pulse series appear, new technology of multi-dimensional spectra and high resolution work on solids.
PRINCIPLE(2, 3, 6):
Nuclei that exhibit the NMR phenomenon are those whichhave the spin quantum number I greater than 0 (I>0). A nucleus with an odd mass or an odd atomic number possess a nuclear spin, due to spinning a magnetic field is generated along the axis. The spin quantum number I of the nuclei as follows:
|
Mass number (A) |
Atomic number (Z) |
Spin quantum number (I) |
|
odd |
odd or even |
1/2, 3/2, 5/2… |
|
even |
even |
0 |
|
even |
odd |
1,2,3, 4….. |
Table-1: The spin quantum number I is associated with the mass number (A) and atomic number (Z) of the nuclei
Without externally applied magnetic field, the nuclear spins are random in all directions. But when externally magnetic field is applied; the nucleus align themselves by creating magnetic momentum.
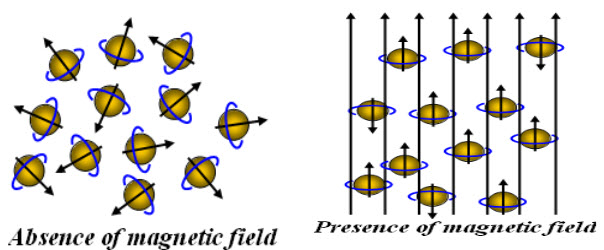
Fig-1: Orientation of spinning nuclei in absence and presence of external magnetic field
Hence, nucleus spins on their own axis when placed in an external magnetic field resulting in a circular motion creating aprecessional orbit, with a frequency called precessional frequency. When energy in the form of radio frequency is applied and is equal to precessional frequency, then the transition of protons from lower energy (α state) to higher energy (β state) take places and NMR signals are recorded.
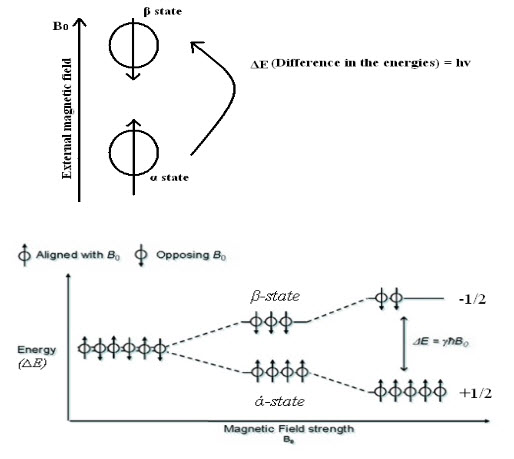
Fig-2: Energy level transitions of protons
When application of radio frequency energy is stopped nucleus returns to ground state. Increasing in strength of magnetic field does not cause transition from lower energy (α state) to higher energy (β state). But it merely increases precessional frequency.
RELAXATION PROCESS(2, 3):
It is the process of transition from excited state to ground state where absorbed radio frequency energy can be lost by two ways:
1.Radiation Emission: lost with emission of radio frequency radiations itself.
2.Radiation Transition: (without radiation) It occurs in two ways:
a) Spin-lattice/ longitudinal relaxation process: where the energy is lost by means of translational/ vibrational/ radiational energy.
b) Spin-spin/ Transverse relaxation process: where the energy is lost to neighboring nuclei.
In a homogenous magnetic field, an atomic nucleus will have (2I+1) orientations. The frequency of radiation needed to flip the proton to higher energy state is
V = Y Ho/2 pi
Where, v = frequency,Ho= Strength of magnetic field (guass), γ = nuclear constant (26750 for protons)
NMR SPECTRUM(3, 8, 10):
The NMR spectrum is a plot of, "intensity of NMR signals Vs frequency (magnetic field)". It is generally calibrated in units of frequency rather than in units of magnetic field strength.
Fig-3: NMR spectrum
INSTRUMENTATION(1, 2, 3, 10):
The Nuclear Magnetic Resonance Spectrophotometers are two types based on the parameters that are measured.
1. Single coil spectrometers: It measures absorption.
2. Double coil spectrometers: It measures resonant radiation.
NMR spectrometers can also be divided into low resolution or high resolution spectrometers, with the former being capable of quantitative element analysis and also being known as wide line spectrometer.The major components of NMR instrument are as follows:
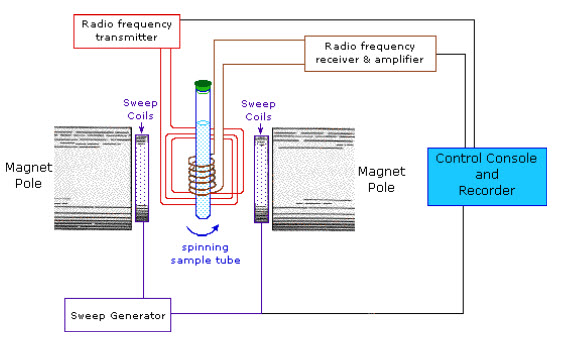
Fig-4: Schematic diagram of NMR Spectrophotometer
Sample Holder:
Usually the dimension of sample holder is 8.5cm in length and 0.3 mmin diameter. Glass tubes are generally used as sample holder as these are more economic. The following ideal characteristics are there in the sample holder.
· It should be sturdy.
· It should be practical.
· It should be cheap.
· It should be transparent to radio frequency radiations.
· It should be chemically inert.
Sample probe:
It holds the sample tube in a magnetic field and rotates it along its axis, resulting in sharper lines with better resolution due to decrease in the effects of in homogeneities in the magnetic field. The probe may be either a single coil or system of coils depending upon the type of instrument.
Permanent Magnet:
Permanent magnet or electromagnet has the important feature that it should give homogeneous magnetic field, i.e., the strength and direction of the magnetic field should not change from point to point. As the field strength is proportional to the chemical shifts, it must not be less than 20,000 guass.
Magnetic Coils:
It is employed for the production of NMR spectra. It is achieved by passing direct current either through the coils that are wound around the magnetic pole or through a pair of Helmholtz coils located on either side of the sample probe. The relationship between the resonance frequency of the nucleus and the strength of magnetic field (H0) is expressed as:
|
v = constant x H0 |
From that equation, frequency is directly proportional to strength of magnetic field (H0). If H0is kept constant, the precession frequency is fixed. If radiofrequency is kept constant, the resonance frequency of the nucleus must be changed by varying H0
Sweep Generator:
If precession frequency is equal to applied frequency radiations, this results in nucleus to resonate. Sweep generator method, is used to vary the magnetic field and it is easier,than the variation of radio frequency.
Radio Frequency Generator:
Radio Frequency Generator is also known as Radio Frequency Transmitter. In order to generate radio frequency radiation, radio frequency oscillator is used which irradiates the sample molecules. Due to applied radiofrequency, an energy difference occurs and the nuclei moves from ground state to excited state. The coil surrounding the sample results in resonance signals.
Radio Frequency Receiver:
When the radio frequency radiation is passed through the magnetized sample two phenomena namely absorption and dispersion may occur. The observation of either absorption or dispersion will enable the resonance frequency to be determined.
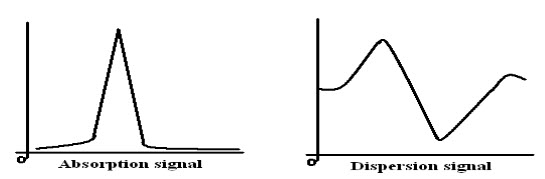
Fig-5: Absorption and Dispersion signals
For the detection of resonance signal following methods are used:
a) Radio Frequency Bridge is employed under single coil instruments. It allows absorption and dispersion signals to appear as an output of EMF across the bridge. Signals can be recorded mechanically.
b) This method employs a separate receiver coil which is sometimes called as crossed coil or nuclear induction method. In this the transmitter and receiver coils are arranged perpendicular to each other and to the direction of the magnetic field.
Amplifier:
The absorption signal received from radio frequency receiver is extremely weak. Therefore, it requires considerable amplification before it is fed to a chart recorder in which amplifier is used for amplification of weak signals.
Read Out:
The NMR spectra obtained from instruments are directly recorded via a computer or even mechanically.
SOLVENT SYSTEM USED IN NMR(3, 10):
A substance which is free of protons should be used as solvent. i.e., it should not give of its own absorption in NMR spectrum. It should be capable of dissolving at least 10% of substance.
Examples:
CCl4 - Carbon Tetrachloride
CS2 - Carbon Disulfide
CDCl3-Deuteriochloroform
C6D6 - HexaDeuteriobenzene
D2O - Deuterium oxide
PARAMETERS EFFECTING NMR SPECTROSCOPY(3,6,9,10):
1.Chemical Shift: A chemical shift is defined as the difference in parts per million (ppm) between the resonance frequency of the observed proton and tetramethylsilane (TMS) hydrogen.Orit is theShifting in positions of NMR absorptions(reference & sample) which arise due to shielding or deshielding of protons by electrons is called as chemical shift.
It is expressed in δ (delta)and τ (tau) scale.

This scale is measured in units of parts per million (ppm) of the spectrometer operating frequency. Tetramethylsilane (TMS) is taken as reference and chemical shifts for different kinds of protons are measured relative to it.
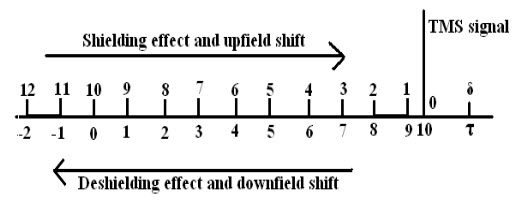
Why Tetramethylsilane (TMS) is taken as reference compound?
It is a chemical compound having a molecular formula Si(CH3)4and tetrahedral structure.
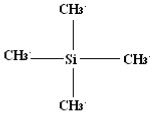
TMS is most convenient reference due to following characteristics:
· All the 12 hydrogen atoms present in TMS remain in an identical environment
· It is inert compound and magnetically isotopic in nature
· It is miscible with all organic substances
· Highly volatile
· Can be easily removed
· Sharp absorption peaks are obtained.
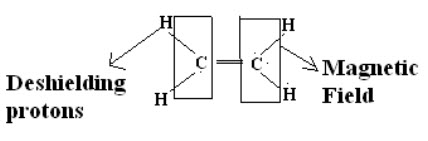
Shielding and Deshielding protons:
If a proton is present inside the circulating magnetic field (due to electron cloud) then it is called as shielding proton (Or) it can also possible if the proton is present nearer to the electropositive atom or a group.
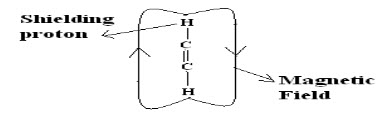
If proton is present on outside of the circulating magnetic field then the proton is said to be deshielding proton (Or) it can also be possible if proton present nearer to electronegative atom or group.
Causes of chemical shift:
When a molecule is placed in a magnetic field its electrons are used to circulate and thus produces secondary magnetic field or induced magnetic field. Rotation of electrons about the proton itself generates a field in such a way that at proton itself opposes applied field.When the induced magnetic field opposes the applied field and proton is said to be shielding.But if induced field reinforces (supports) the applied field proton feels higher field strength and such a proton is said to be in deshielding.
Factors influencing Chemical Shift:
The following are the factors which affects chemical shift:
a) Inductive effect
b) Vander waals deshielding
c) Anisotropic effect
d) Hydrogen bonding
a)Inductive effect:
Inductive effect simply means development of polarity. The presence of an electronegative group in an atom deshields the proton. As the electronegativity of an atom increases the deshielding effect in the proton also increases and hence the δ value also increases.
E.g.:
|
Molecule |
δ value |
|
CH3-CH3 |
0.9 |
|
CH3-I |
2.2 |
|
CH3-Ar |
2.7 |
|
CH3-Cl |
3.06 |
|
CH3-F |
4.3 |
b)VanderwaalsDeshielding:
In overcrowded molecules it is possible that some proton occupy stearically hindered position. Electron cloud of bulky groups will tend to repel electron cloud surrounding the proton.Such a proton will be deshielded and will resonate at higher value ofδ.
c)Anisotropic Effect:
It is also known as space effect. Anisotropic effects constitute shielding and deshielding of protons because of induced magnetic field. Aldehydic and aromatic protons are more deshielded. Alkyne protons appear at relatively low value of δ (shielding).
d)Hydrogen Bonding:
The presence of hydrogen bonding in a molecule results in the shifting of proton signals towards down field which revealsdeshieldingprotons. Electronegative atoms like F, N,andO attached to hydrogen bonding proton results in attraction of electrons towards F,N and O leading to decrease electron density around the proton.
2. Spin Spin Coupling:
The interaction between the spins ofneighbouringnuclei in the molecule may causes splitting of NMR. This is called as spin spin coupling. This is related to the number of possible combinations of the spin orientations of the neighboring protons.
Splitting pattern: (how many neighboring hydrogen’s), In general, n-equivalent neighboring hydrogen’s will split a 1H signal into an (n + 1)
Pascaltriangle pattern.
|
n |
n + 1 |
Pascal pattern: |
Multiplicity |
|
0 |
1 |
1 |
Singlet (s) |
|
1 |
2 |
1:1 |
Doublet (d) |
|
2 |
3 |
1:2:1 |
Triplet (t) |
|
3 |
4 |
1:3:3:1 |
Quartet (q) |
|
4 |
5 |
1:4:6:4:1 |
Quintet |
Table-2: Pascal triangle pattern
This is explained by comparing the NMR spectrum of benzyl acetate with that of ethyl formate exhibits not only difference in the postion of the resonance signals but also a difference in the multiplicity of the signals.
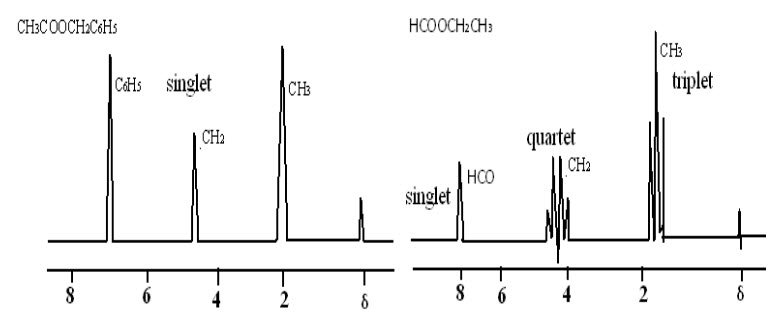
Fig-7: NMR spectra of benzyl acetate and ethyl formate
In case of benzyl acetate, singlets are observed for methyl (CH3) and methylene (CH2) protons while in ethyl formate the same protons give rise to a triplet and quartet. This is mainly due to spin spin coupling.
Rules of Spin Spin Coupling:
· Chemically equivalent nuclei do not show spin-spin coupling.
E.g.: CH4, CH3-CH3give only a single resonance line
· Only non-equivalent protons couple.
· Protons on adjacent carbon normally couple.
· Protons separate by four or more bonds will not couple.
3. COUPLING CONSTANT (J):
The distance between the adjacent lines of multiplet is a measure of splitting effect known as coupling constant. It is expressed in Hz.Coupling constant is a measure of spin spin coupling.
E.g.: In case of freely rotating groups with anti-conformation have J = 5 – 12 Hz while protons with gauche conformation have J = 2 – 4 Hz.
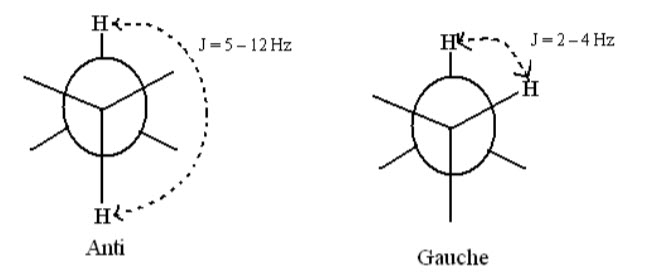
In case of vinylic protons, where restricted rotation due to double bonds is involved the cis protons have J = 6 – 14 Hz whereas trans protons have J = 11 – 18 Hz.

Factors influencing coupling constant:
· Coupling through multiple bonds can occur over greater distance because of mobility of π electron.
E.g.: coupling values of olefinic protons decrease in order of trans>cis> gem.
· In aromatic system ortho coupling >meta coupling >para coupling.
· Coupling between germinal protons on saturated carbons depends on bond angle.
J value increases with increasing in bond angle.
· Increasing in electro negativity causes decrease in J value.
INTERPRETATION OF NMR SPECTRA(2, 3, 10):
NMR spectrum of a substance gives very valuable information about its molecular structure. This information is gathered as follows:
1. The number of signals in the PMR spectrum tells us how many kinds of protons in different chemical environments are present in the structure under examination.
2. The position of signals gives information about each kind of protons.
3. The intensities of different signal give information about the presence of relative number of protons of different kinds.
4. The splitting of signals tells us about the absorbing proton with respect to neighbouring protons.
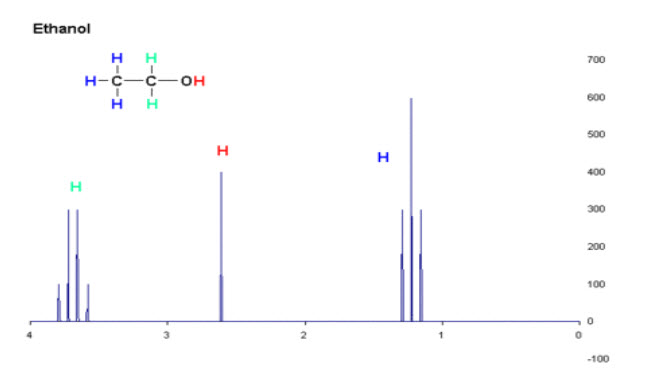
Fig-8: NMR Spectrum of Ethanol
When the spectrum is recorded for ethanol it gives Triplet, quartet and singlet peak because of the presence of three kinds of protons.The triplet is because of three equivalent methyl protons and splits into a triplet because of the two neighbouring hydrogen’s on methylene group. The quarter is for two methylene protons and multiplicity of four peaks is due to coupling with the three methyl protons but the hydroxyl proton will not couple. Finally the singlet peak is due to hydroxyl proton which does not show any coupling with adjacent methylene protons.
APPLICATIONS(3, 4, 7, 8, 10):
The two major areas where NMR has proven to be of critical importance are in the fields of medicine and chemistry, with new applications being developed daily.
1. Chemistry related applications:
I.Structural diagnosis:
A large number of principles are known which will decide about structure of an unknown from its NMR spectrum. Some of these are outlined as follows:
· The number of main NMR signals should be equal to number of equivalent protons in unknown compounds.
· Chemical shift indicates types of H-atoms present.
E.g.: Methylene, Methyl, Ethers etc.
· Spin-spin splitting or multiplicity reveals possible arrangement of groups in molecule.
· From the area of peaks; number of hydrogen nuclei present in each group can be determined.
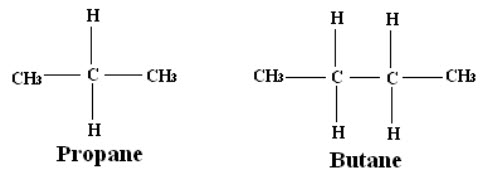
E.g.: The ratio of areas of methyl (CH3) and methylene (CH2) peaks in the propane and butane are 6:2 and 6:4 respectively.
II.Quantitative Analysis:
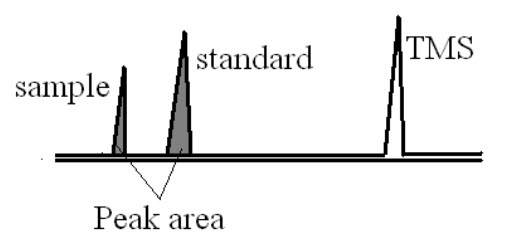
a)Assay of component or % purity:
This method is used to determine % purity of active pharmaceutical ingredient. In this standard and sample compounds are taken and NMR spectra are recorded. From that spectrum by comparing the peak area of both sample and standard the % purity of the compound is determined.
b)Chain length of surfactants
By examining the NMR spectra, it is easy to determine the chain length of the surfactant.
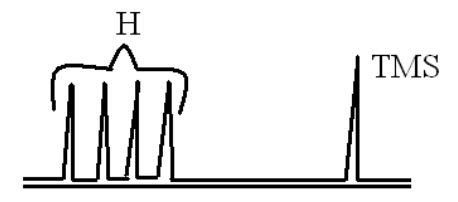
If we observe the spectrum of the above compound it contains four peaks which indicates that the compound contain four typeof hydrogen’s.From this we can identify the chain length of the surfactants.
c)Hydrogen analysis:
By using spectrum of any compound we can easily determine how many number of hydrogen’s present in each compound.
d)Moisture analysis:
The molecular formula of Water contains two hydrogen atoms and one oxygen atom. The hydrogen is deshielded due to the attachment of strongly electronegative atom. For such deshieldingthe absorption peak is shifted to downfield.
e)Hydrogen Bonding:
NMR has been used in a simple way to distinguish between intermolecular and intramolecular hydrogen bonding including chelation. As the hydrogen bonding involves electron cloud transfer from the hydrogen atom to a neighbouring electronegative atoms like F, N and O, the hydrogen experience a deshielding and shift to downfield.
III.Determination of Keto-EnolTautomerism:
E.g.: Acetyl acetone
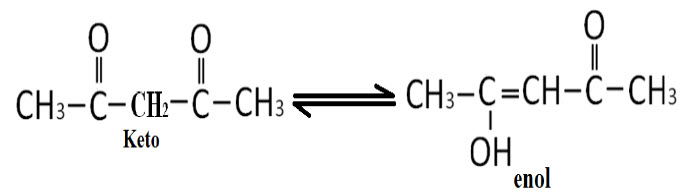
Keto-Enol form of any compound can be identified by NMR spectra, according to the peaks count
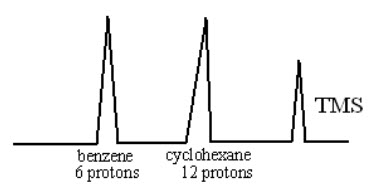
IV.Determination of molar composition of the compound:
Consider an example of mixture containing both cyclohexane and benzene. As one mole of cyclohexane contains 12 protons and benzene contains 6 protons. If the peak area of both benzene & cyclohexane are same then it is confirm that two mole of benzene and one mole of cyclxexane are there in the mixture.
V. Determination of Geometrical Isomers:
Geometrical isomers can be distinguished by observing different chemical shift values and their coupling constant values.
Cis form → J value → 7 - 12
Trans form → J value → 13 - 18
The values of coupling constants vary for axial and equatorial positions of the proton groups, there by cis and trans forms can be distinguished easily.
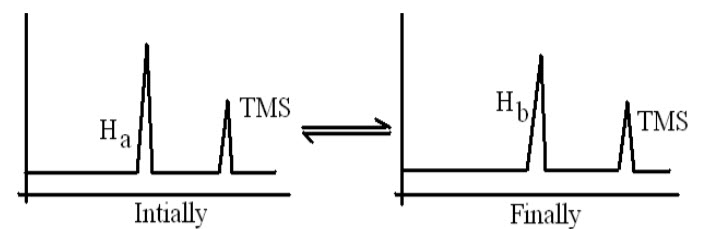
VI.Identification of chemical reaction:
NMR is used to identify whether the chemical reaction is complete or not.
E.g.:CH3OOHa + HOHb <-----> CH3COOHb+ HOHa
VII.Determination of activated energy:
NMR is used for the determination of activation energy.
E.g.: N, N – dimethyl acetamide (DMA)
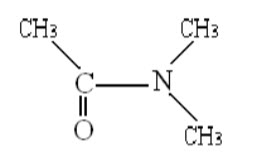
Spin-spin coupling between protons on different methyl groups is absent due to large separation. At a temperature of -300C, the nuclear magnetic resonance spectrum of DMA shows both peaks and a doublet, indicating that the two methyl groups attached to the nitrogen are not equivalent. Magnetic equivalence of these methyl groups can be realized by heating the compounds. Thus as the temperature of DMA is increased, the peaks of the doublet begin to broaden and ultimately convert into singlet at 900C. From that activation energy () is calculated using
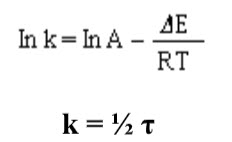
VIII.Study of Isotopes other than Protons:
Nuclei having magnetic moments in addition to that of proton can be studied by using the nuclear magnetic resonance spectroscopy.
E.g.: fluorine and phosphorus. The 31P bearing spin number ½ exhibits sharp nuclear magnetic resonance peaks, with a resonance frequency of 24.3 MHz at 1400 guass.
2. Medical related applications:
NMR is used in medical field for diagnosis of human body in two ways:
· Used for diagnosis for inborn errors of metabolism in urine like, phenyl ketonuria, Maple's urine syndrome.
· Magnetic resonance imaging (MRI) is an important medical diagnostic tool used to study the function and structure of the human body which is very safe to administer.
3.Other applications:
NMR has also proven to be very useful in other fields such as non destructive testing, data acquisition in the petroleum industry, process control, earth’s field NMR and magnetometers.
· Non-destructive testing saves a lot of money for expensive biological samples and can be again used if more trials are needed to perform.
· The petroleum industry uses NMR equipment to measure porosity of different rocks and permeability of different underground fluids.
· Magnetometers are used to measure the various magnetic fields that are relevant.
LIMITATIONS(2, 3, 9, 10):
· It is less sensitive
· Overlapping of spectra occur which make difficult to analyze the desired spectra.
· Molecular weight of compound cannot be determined.
· It is Expensive.
· Large amount of sample is required.
· Mainly used for analysis of liquid compound.
ADVANTAGES(3, 4, 8):
· It is most powerful technique used for the examination of nuclear structure.
· Simple, sample preparation prior to actual measurement.
· A wide variety of organic and inorganic compounds can be analyzed.
· Used for identification of compounds using fingerprint technique.
13C NUCLEAR MAGNETIC RESONANCE(2, 3, 10)
Carbon – 13 or 13C NMR was first studied in the year 1957 but it was not in the scenario until the early 1970’s due to the lack of advancement in the development of sensitive instruments to detected weak signals exhibited by C – 13 nucleus.
Carbon – 13 spectra are inherently less complex than proton spectra for two reasons:
a) Chemical shift between 13C nuclei in different chemical environments can differ by as much 200 ppm where proton shift differences are seldom more than 10 ppm.
b) As the natural isotopic abundance of 13C is only 1.1% coupling.
The principle governing 13C NMR spectroscopy is exactly similar to those of the proton NMR spectroscopy. It also employs tetramethylsilane (TMS) as the common reference compound and the chemical shifts are usually expressed in dimensionless δ units (ppm).
Coupling between adjacent carbons is not generally observed 13C NMR spectra are usually acquired in a mode that removes the effect of any coupling to the protons attached to the carbons.
APPLICATIONS(2,3):
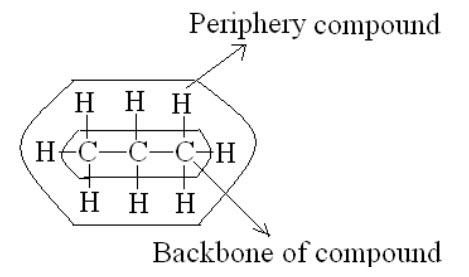
I. We can get information about the backbone of the compounds but not the periphery.
E.g.:
II. Basically chemical shift range is upto 0 – 200 ppm. If we get chemical shift in this region no chance of overlapping of spectrum.
III. Spin spin splitting is not possible so that clear spectrum is observed.
IV. Hetronuclear spin coupling between isotopes i.e.13C and 12C is not possible because 12C has pair of electrons
V. Individual resonances can also be detected for each carbon atom in a molecule if the molecular mass lies in between 200 – 400.
VI. The homonuclear spin spin coupling between carbon atoms is not possible as the probability for the occurrence of two 13C atoms adjacent to each other is very small.
19F NUCLEAR MAGNETIC RESONANCE(3)
Fluorine – 19 has a spin quantum number of ½ and a magnetic moment of 2.6285 nuclear magnetons. Thus the resonance frequency of fluorine is 56.4 MHz and only slightly lower than that of the proton with 60.0 MHz at 14092 G.Fluorine absorption is experimentally found to be sensitive this results chemical shifts extended over a range of about 300 ppm compared with a maximum of 20 ppm for the proton.
31P NUCLEAR MAGNETIC RESONANCE(2,3)
Phosphorus – 31 with spin number ½ also exhibits sharp NMR peaks with chemical shifts extending over a range of 700 ppm. The resonance frequency is 24.3 MHz at 14,092 G.
CONCULSION:
The power of NMR is to "visualize" how molecules interact, in the context of molecule dynamic and three dimensional structure make it unique for studying the mechanistic and functional properties of proteins and nucleic acids. In this no sample preparation, which saves time and effort and Calibration is easy and straight forward, delivering accurate and precise results. Over the past few decades, NMR has a very dynamic and exciting area. So, I truly believe that this is a trend that will stay for decades to come.
REFERENCES:
1.U. Holzgrabe, I. Wawer, B. Diehl; A text book of NMR spectroscopy in Pharmaceutical analysis; Page. No: 277-279.
2.B. K. Sharma; A text book of instrumental methods of chemical analysis;Page.No:619 - 736.
3.Gurdeep R. Chatwal, Sham K. Anand; A text book of instrumental methods of chemical analysis; Page. No: 2.185-2.220.
4.Willard, Merritt Dean; seltte; Instrumental method of analysis; 6th edition; Page. No: 422 – 454.
5.J. Mendham, RC Denney, JD. Barner, MJK Thomas; text book of qualitative chemical analysis; 6th edition; Page. No: 599 – 611.
6.Y.Anjaneyulu, K.chandraSekharValliManikam; A Text book of Analytical Chemistry; Page. No: 682 – 712.
7.Kenneth A. Connors; A Textbook of Pharmaceutical Analysis; 3rd edition; Page. No:279 – 301.
8.Anees A. Siddiqui; Pharmaceutical Analysis: volume II; Page. No: 99-131.
9.Dr. A.V. Kasture, Dr.S.G.Wadodkar, Dr.K.R.Mahadik, Dr.H.N.More; Pha









