 About Authors:
About Authors:
Shashi Kant*, Dr. Bharat Prashar
Department of Pharmaceutical Sciences,
Manav Bharti University,
Solan (H.P)
*shashi_ranaute@yahoo.in
ABSTRACT:
In this review article, we discussed abouteffluent testing and treatment in pharmaceutical industry,Biological treatment of wastewater is frequently the most beneficial method for selecting various toxic compounds from the environment. Most of the organic compounds in industrial wastewaters are of natural origin and can be degraded by common bacteria in aerobic or anaerobic processes. The composition of these wastewaters is very variable. A great variety of organic chemicals can be determined among the principal components of these types of wastewaters. Antibiotics are the major group of pharmaceuticals. Among all the other pharmaceutical drugs and substances, antibiotics are important compounds due to its serious irreversible increase the release to the environment[1][2][3][4][5]
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1305
INTRODUCTION:-
Biological treatment of wastewater is frequently the most beneficial method for selecting various toxic compounds from the environment. Most of the organic compounds in industrial wastewaters are of natural origin and can be degraded by common bacteria in aerobic or anaerobic processes. “Due to its proven capacity to degrade certain toxic components as well as most common organic pollutants in industrial wastewater, anaerobic treatment today has advanced to a high level of usefulness in the restoration of many industrial effluents” ( Donlon, Flores, Field & Lettinga, 1995). The pharmaceutical industry constitutes from the wastewaters containing toxic organic chemicals. The composition of these wastewaters is very variable. A great variety of organic chemicals can be determined among the principal components of these types of wastewaters. Antibiotics are the major group of pharmaceuticals. Among all the other pharmaceutical drugs and substances, antibiotics are important compounds due to its serious irreversible increase the release to the environment. Antibiotics are in effluent of treatment plants and receiver media due to difficult treatability with conventional treatment systems such as aerobic active sludge system. For that reason, they spoil ecological balance to form toxicity to organisms in ecosystem and biological treatment systems.
Objectives and Scopes
The purpose of this study is to provide the treatability of two types of antibiotics (Kemicetine and Sulfamerazine) in an up flow anaerobic sludge blanket (UASB) reactor / aerobic completely stirred tank (CSTR) reactor and an anaerobic baffled (ABR) reactor / aerobic (CSTR) reactor systems. There is not enough knowledge about the treatability of pharmaceutical wastewater in anaerobic conditions. Furthermore, no study about the treatability of wastewaters containing antibiotic was encountered using both UASB and ABR reactors. Therefore this thesis was designed to investigate these lacks in the literature. The major objectives of this research can be summarized as follows;
1. to investigate the removal efficiencies COD and BOD5, VFA production, total and methane gas productions of synthetic wastewaters containing antibiotic in different Kemicetine and Sulfamerazine doses in sequential UASB/ CSTR and ABR/ CSTR reactor systems,
2. to monitor the toxicity of synthetic wastewater containing antibiotic based on specific methanogenic activity (SMA) tests,
3. to characterize the wastewater composition based on inert COD, slowly and readily biodegradable organic substances and BOD5 / COD ratios,
4. to compare both systems according to their treatment efficiencies.
[adsense:468x15:2204050025]
Physiochemical measurement:-
Temperature
Measured using pH and dissolved oxygen meter, or thermometer.
pH
“acidity” of the water, measured using a pH meter. This meter is used to measure the acidity of the water by comparing readings from a reference electrode and a sample electrode. To determine pH the output of these electrodes must be temperature-compensated, most pH meters also measure temperature.
Turbidity
The clarity of the water, measured using a portable turbidimeter. The turbidimeter measures the light transmittance of a sample in NTU's (Nephelometric Turbidity Units, a standard measure). It needs no field calibration. Handle the sample vials only by their ends (preferably the lid) so as not to affect the transmittance; wipe any fingerprints, spots, etc. from the outside of the vial; and be sure to close the vial-compartment lid when taking a measurement.
DO
Dissolved Oxygen content. Measured using a hand-held dissolved oxygen meter.
The DO meter will measure dissolved oxygen, electrical conductivity, and salinity. DO is measured by the rate of consumption of oxygen at the tip of the probe, so it requires continual movement of water past the tip (an up-and-down motion seems to work best, keep the probe tip submerged). Stable readings are not possible while the temperature of the sample is changing. When performing analysis at multiple sampling locations, the DO meter calibration should be checked at the beginning, middle, and end of each analysis day. Oxygen from the atmosphere enters water through adsorption at its surface, a process enhanced by agitation (rapid moving water). In flowing water, oxygen-rich water at the surface is constantly being replaced by water containing less oxygen as a result of turbulence, creating a greater potential for exchange of oxygen across the air-water interface. Flowing water is more likely to have high DO levels than stagnant water Because still water undergoes less internal mixing, the upper layer of oxygen-rich water tends to stay at the surface, resulting in lower dissolved oxygen levels throughout the rest of the water levelsDO can also enter water as a waste product of photosynthesis by aquatic plants, a process highly dependant on sunlight.The amount of oxygen that can be held by water depends on the water temperature, salinity, and pressure.Gas solubility increases with decreasing temperature and decreasing salinityColder water can hold more oxygen than warm water and freshwater can hold more oxygen than saltwater.Gas solubility decreases as pressure decreases so the amount of oxygen absorbed in water decreases as altitude increases.
Winkler method :-
The Winkler method for the determination of dissolved O2 involves adding an excess of ‘white’ Mn(OH)2 which contains Mn2+ ions.
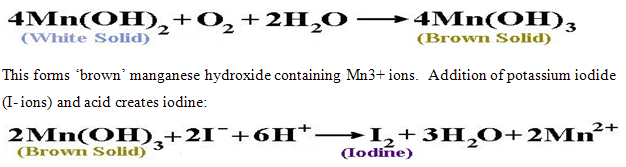
For every molecule of O2 two molecules of I2 are formedThe quantity of iodine present can be found though titration of the sample with sodium thiosulphate.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Biochemical oxygen demand or BOD :-BOD refers to the amount of oxygen th









