 ABOUT AUTHOR:
ABOUT AUTHOR:
Sunitha Manthena
Nalla Narsimha Reddy School of Pharmacy,
Narapally, Hyderabad
Affiliated to JNTU(H);
sunithatgk@gmail.com
INTRODUCTION
The expenses for developing new drugs are exorbitant. Thus in the present scenario, more emphasis is laid to develop newer drug delivery technologies, which would ensure better patient compliance, drug efficacy and extends the term of patents of the existing molecules. In the present era, the pharmaceutical industry is facing several challenges due to worldwide competition and growing demand for better products.1
For the purposes of drug delivery, the Colon has to be considered as two regions; the proximal Colon and the distal Colon. Formulations targeted to the proximal Colon have to be delivered via the oral route and must be protected against the hostile environment of the stomach and small intestine. Formulations targeted to the distal Colon can be reached through the anus by rectal route is preferable for drugs which produce emesis or gastric irritation when given orally. Transit through the Colon is slower than other areas of the gastrointestinal tract and so there is an opportunity for sustained drug delivery from the ascending and first part of the transverse Colon.2
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1734
1.1 Colon-specific drug delivery system
Colonic delivery refers to targeted delivery of drugs into the lower GI tract, which occurs primarily in the large intestine (i.e. Colon). The site-specific delivery of drugs to lower parts of the GI tract is advantageous for localized treatment of several Colonic diseases, mainly inflammatory bowel disease (Crohn’s disease and ulcerative colitis), irritable bowel syndrome, and Colon cancer. Other potential applications of Colonic delivery include Chronotherapy, prophylaxis of Colon cancer and treatment of nicotine addiction2, 3. It has also gained increased importance not just for the delivery of drugs for the treatment of local diseases4, but also potential site for the systemic delivery of therapeutic proteins and peptides which are being degraded or poorly absorbed in the upper gut, such as peptides and proteins, may be better absorbed from the more benign environment of the Colon. These delivery systems when taken orally, allows the drug to release from the delivery system once it arrives into the Colon1.
Colon targeted drug delivery would ensures direct treatment at the disease site, to improve the efficacy of the drug by concentrating the drug molecules where they are needed most, and also minimizes the potential side effects and drug instability issues associated with premature release of drug in the upper parts of the GIT, namely stomach and small intestine4, 5.
1.1.1 Anatomy of Colon 5
The GI tract is divided into stomach, small intestine and large intestine.The large intestine is wider and shorter than the small intestine.The large intestine extending from the ileo-cecal junction to the anus is divided in to three main parts as shown in Figure 1.0. These are the Colon, the rectum and anal canal.
The entire Colon is about 5 feet long, and is divided into five major segments.The cecum forms the first part of the Colon and leads to the right Colon or the ascending Colon (just under the liver) followed by the transverse Colon, the descending Colon, sigmoidal Colon, rectum, and the anal canal. Peritoneal folds called as mesentery which is supported by ascending and descending Colon. The right Colon consists of the cecum, ascending Colon, hepatic flexure and the right half of the transverse Colon. The left Colon contain the left half of the transverse Colon, descending Colon, splenic flexure and sigmoid. The rectum is the last anatomic segment before the anus. 2, 5 Unlike the small intestine, the Colon does not have any villi. However, because of the presence of plicae semilunares, which are crescentic folds, the intestinal surface of the Colon is increased to approximately 1300 cm2. The Colon is a cylindrical tube lined by a moist, soft pink lining called mucosa; the pathway is called the lumen and is approximately 2 to 3 inches in diameter.6
[adsense:468x15:2204050025]
1.1.2 Physiology of Colon 5, 6
The physiology of the proximal and distal Colon differs in several respects that have an effect on drug absorption at each site. The physical properties of the luminal content of the Colon also change, from liquid in the cecum to semisolid in the distal Colon.
The major functions of the Colon are:
1) The consolidation of the intestinal contents into feces by the absorption of the water and electrolytes and to store the feces until excretion. The absorptive capacity is very high; each day about 2000 ml of fluid enters the Colon through the ileo-cecal valve from which more than 90% of the fluid is absorbed
2) Creation of a suitable environment for the growth of Colonic microorganisms, such as Bacteroides, Eubacterium and Enterobacteriaceae.
3) Expulsion of the contents of the Colon at a suitable time.
4) Absorption of water and Na+ from the lumen, concentrating the fecal content, and secretion of K+ and HCO3-.
5) The active secretion of K+ is stimulated by mineralo-corticoids.
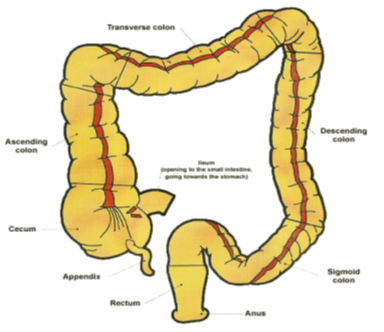
Figure 1.0: Anatomy of Colon
1.1.3 Factors to be considered in the design of Colon-specific drug delivery systems
Various factors affect the absorption of the drug molecules from the Colon. These factors include physiological, pathological and pharmaceutical factors. The physiological factors that influence the bioavailability of Colonic delivery dosage forms include:
* Barriers in Colonic absorption
* Gastrointestinal transit
* Gastric emptying time
* pH along the GI tract
* Colonic micro flora
The pathological states also have pronounced effect on Colonic absorption of drug molecules by affecting the Colonic transit e.g. diarrhoea will result in increase in the gastric motility and constipation results in decrease in Colonic motility.
1.1.3.1 Barriers in Colonic absorption
Drug absorption from the Colon can be limited by a number of barriers. In the lumen itself, specific and non specific drug binding occurs through the interaction of the drug with dietary components11. The mucus layer at the epithelial surface, due to its highly charged and sieve-like nature, presents a formidable thermodynamic barrier to the transit of large, negatively-charged drug molecules. This might facilitate longer Colonic residence time and hence environmental or enzymatic degradation. Although removal of the mucus barrier using mucolytic agents might seem attractive, this may implicate in a variety of disease processes and pathological conditions due to alteration of intact mucus layer.
Another physical barrier to drug absorption, is at the level of epithelium, the drugs intending to pass from the epical to barolateral surface of the epithelial barrier must do by passing through either Colonocytes (the transcellular route) or between adjacent Colonocytes (the paracellular route).
Drug Absorption from the Colon: Drug absorption in the Colon takes place by two routes:
* Paracellular Route
* Transcellular Route
Transcellular absorption involves passage of the drugs through cells and this is the route for lipophilic drugs takes, whereas paracellular absorption involves the transport of drug through the tight junctions between cells and is the route for most of the hydrophilic drugs takes. The poor paracellular absorption of many drugs is due to the fact that the epithelial cell junctions are very tight.
Colon is a more selective site for the drug absorption than small intestine for many drugs. Drugs shown to be well absorbed include diclofenac, ibuprofen, and theophylline. Drugs shown to be less absorbed include atenolol, cimetidine, hydrochlorthiazide, lithium.
Carrier-mediated absorption in the Colon is not extensive and usually relates to the metabolic events in the residual bacteria. The movement of peptides is predominately dependent on the hydrogen bonding. Compared with peptides, proteins experience a greater thermodynamic barrier for passing through cell membranes and more vulnerable to denaturation at the interface. Drug molecules that have traversed the physical and enzymatic barriers of the Colonic mucosa may enter either the blood capillary bed or the lymphatic sinuses. Intact drugs that reach the venous capillaries from the sub mucosa are transported to the liver via the hepatic-portal system where they undergo significant metabolism 6, 7.
Factors affecting the absorption of the drug molecules from the Colon15
- Physical characteristics of drug (pKa, degree of ionization of drugs)
- Colonic residence time as dictated by gastrointestinal tract motility.
- Degradation by bacterial enzymes and by products.
- Selective and nonselective binding to mucus
- Local physiological action of drug
- Disease state
- Use of chemical absorption enhancers, enzyme inhibitors, or bioadhesives.
Colonic absorption of drug molecules may be improved by using following strategies:
* Co-administration of absorption enhancers.
* Inhibition of proteolytic enzymes in the Colonic lumen.
The permeability of the epithelium to the drugs can be enhanced by the use of chemical enhancers, which promote the absorption. A wide range of compounds have been used as absorption enhancers. These are calcium ion chelating agents (EDTA), surfactants, bile salts and fatty acids as shown in Table 1.0.
These enhancers increase transcellular and paracellular transport through one or other following mechanisms:
* By disruption of intercellular occluding junction complex function to open t
* By disrupting the integrity of lipid bilayer of Colonic enterocytes.
* By modifying the epithelial permeability via denaturing membrane proteins.
Enhancement of the Colonic-absorption by these agents appears to be drug-specific e.g. mixed micelles composed of either glycocholate or taurocholate, mono olein, oleic or lauric acid have shown to enhance the absorption of 5-fluorouracil, heparin and bleomycin.
1.1.3.2 Gastrointestinal transit
Gastric emptying of dosage forms is highly variable and depends primarily on whether the subject is fed or fasted and on the properties of dosage form such as size and density. The presence of food generally increases gastric residence and in some cases, with regular feeding, dosage forms have been shown to reside in the stomach for periods in excess of 12 h. Small intestine transit is surprisingly constant at 3-4 hrs and appears to be independent of the physical state, size of dosage form, presence of food in the stomach. Table 1.1 shows the approximate gastrointestinal transit time of contents.
Compared to other regions of the gastrointestinal tract, movement of the materials through the Colon is slow. Colonic transit time is only slightly affected by food but is reduced under stress. The total time for transit tends to be highly variable and influenced by number of factors such as diet, in particular dietary fiber content, mobility, stress, disease and drugs. Studies have shown that drugs that act on the parasympathetic or sympathetic nervous system affect the propulsive motor activity, thereby influencing the Colonic transit time. 10, 12
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1.0: Compounds demonstrating enhancement of Colonic drug absorption 28
|
Class |
Examples |
|
Chelating agents |
EDTA Citric acid |
|
NSAIDS |
Indomethacin Salicylates |
|
Surfactants |
Polyoxyethylene lauryl ether Saponin |
|
Bile salts |
Taurocholate Glycocholate |
|
Fatty acids |
Sodium caprate Sodium caprylate Sodium laurate Sodium oleate |
|
Mixed micelles |
Monolein-taurocholate Oleic acid-taurocholate Oleic acid-polyoxyethylene hydrogenated- castor oil(HCO 60) Oleic acid-glycocholate |
1.1.3.3 pH along GI tract
There is large variation of pH along entire GI tract. In the stomach pH ranges between 1 and 2 during fasting but increases after eating. Once the dosage form is discharged from the stomach it reaches small intestine where pH ranges from 6.5 in the proximal part and about 7.5 in the distal portion.
From the ileum to the Colon pH declines significantly, it is about 6.4 in the caecum. However, pH values as low as 5.7 have been measured in the ascending Colon in healthy volunteers. The pH in GI tract is subjected to both inter and intra-subject variations.Table 1.1 gives an overview of pH in GI tract.14
Radio-telemetry has been used to measure the gastrointestinal pH in healthy human subjects. The fall in the pH on entry into Colon is due to the presence of short chain fatty acids arising from the bacterial fermentation of polysaccharides. Consequently polysaccharide drugs and diet can affect the Colonic pH. Colonic pH is reduced in disease state.13
Table 1.1: Gastrointestinal Transit time of contents and pH
|
Organ |
Transit Time (hr) |
pH |
|
Stomach |
<1(fasting) >3(fed) |
1.2 |
|
Small intestine |
3-4 |
5.8 - 6.8 |
|
Large intestine |
20-30 |
7.2 -7.5 |
1.1.3.4 Colonic micro flora
A large number of anaerobic and aerobic bacteria are present throughout the entire length of human GI tract. Over 400 species of bacteria are found in the Colon, which are predominantly anaerobic such as Bacteroids, Bifidobacterium, Eubacterium and Clostridium and a small number of fungi. The bacterial count (CFU/mL) in different regions of gastrointestinal tract is tabulated in Table 1.2. The rate of microbial growth is of greatest in proximal areas because of high concentration of energy source. The principal source of nutrition for the Colonic microorganisms is carbohydrates. The carbohydrates are degraded by the action of polysaccharidase and glycosidase enzymes and the ultimate products of fermentation are short chain fatty acids, carbon dioxide, hydrogen, methane and hydrogen sulphide. The bacteria within the Colon are predominantly anaerobic and there is a low reducing environment (low reducing potential).1
Table 1.2: Bacterial count in different regions of GIT
|
GI Region |
Bacterial count (CFU/mL) |
|
Stomach |
0-103 |
|
Jejunum |
0-105 |
|
Ileum |
103-107 |
|
Colon |
1011-1012 |
1.1.4 GENERAL CONSIDERATIONS FOR DESIGN OF COLONIC FORMULATIONS
Formulations for Colonic delivery are, in general, delayed-release dosage forms which may be designed either to provide a ‘burst release’ or a sustained / prolonged release once they reach the Colon.7 The proper selection of a formulation approach is dependent upon several important factors, which are listed below:
(a) Pathology and pattern of the disease, especially the affected parts of the lower GI tract or physiology and physiological composition of the healthy Colon if the formulation is not intended for localized treatment.
(b) Physicochemical and biopharmaceutical properties of the drug such as solubility, stability and permeability at the intended site of delivery.
(c) The desired release profile of the active ingredient.
The most common physiological factor considered in the design of delayed release Colonic formulations is pH gradient of the GI tract. In normal healthy subjects, there is a progressive increase in luminal pH from the duodenum (pH = 6.6 + 0.5) to the terminal ileum (pH = 7.5 + 0.4), a decrease in the ceacum (pH = 6.4 + 0.4), and then a slow rise from the right to the left Colon with a final value of 7.0 + 0.7. Some reports suggest that alterations in GI pH profiles may occur in patients with inflammatory bowel disease, which should be considered in the development of delayed release formulations.8
Formulation of drugs for Colonic delivery also requires careful consideration of drug dissolution and release rate in the Colonic fluids. Generally, the dissolution and release rate from Colonic formulations is thought to be decreased in the Colon, which is attributed to the fact that less fluid is present in the Colon than in the small intestine. The poor dissolution and release rate may in turn lead to lower systemic availability of drugs. These issues could be more problematic when the drug candidate is poorly water-soluble and require higher doses for therapy. Consequently, such drugs need to be delivered in a presolubilized form,or delivery should be directed to the proximal Colon, as a fluid gradient exists in the Colon with more free water present in the proximal Colon than in the distal Colon9.
The stability of the drug in the Colonic environment is a further factor that warrants attention. The drug could bind in a non-specific manner to dietary residues, intestinal secretions, mucus or general faecal matter, thereby reducing the concentration of free drug. Moreover, the resident micro-flora could also affect Colonic performance via degradation of the drug 1, 5.
Table 1.3: Criteria for selection of drugs for CDDS
|
Criteria |
Pharmacological class |
Non-peptide drugs |
Peptide drugs |
|
Drugs used for local effects in Colon against GIT diseases |
Anti-inflammatory drugs |
Ibuprofen, Isosorbides, Theophylline |
Amylin, Antisense oligonucleotide |
|
Drugs poorly absorbed from upper GIT |
Antihypertensive and Anti-anginal drugs |
Oxyprenolol, Metoprolol, Nifedipine |
Cyclosporine, Desmopressin |
|
Drugs for Colon cancer |
Antineoplastic drugs |
Bromophenaramine, 5-Flourouracil, Doxrubicin |
Epoetin, Glucagon |
|
Drugs that degrade in stomach and small intestine |
Peptides and Proteins |
Pseudoephedrine |
Gonadoreline, Insulin, Interferons |
|
Drugs that undergo extensive first pass metabolism |
Nitroglycerin and Corticosteroids |
Bleomycin, Nicotine |
Protirelin, Sermorelin, Saloatonin |
|
Drugs for targeting |
Anti-arthritic and Anti-asthamatic drugs |
Prednisolone, Hydrocortisone, 5-Amino-salicylic acid |
Somatropin, Urotoilitin |
1.1.5 Strategies for Colon-specific drug delivery
Different approaches have been studied for the purpose of achieving Colonic targeting and are summarized below. Targeted drug delivery is reliant on the identification and exploitation of a characteristic that is specific to a target organ. In the context of Colonic targeting, the exploitable GI tract characteristics are pH, transit time, pressure and bacterial flora.
[A] Primary approaches for CDDS 17
(a) pH dependent systems
(b) Time dependent drug delivery to Colon
(c) Microbially triggered drug delivery to Colon
* Prodrug approach for drug delivery to Colon
* Azo-polymeric approach for drug delivery to Colon
* Polysaccharide based approach for drug delivery to Colon
[B] Newly developed approaches for CDDS 16, 17
(a) Intestinal-Pressure controlled drug delivery system (IPCDCS)
(b) CODES™ (A Novel Colon targeted delivery system)
(c) Osmotic controlled drug delivery to Colon (OROS-CT)
(d) Colonic drug delivery system based on pectin and galactomannan coating
[A] Primary approaches for CDDS
(a) pH dependent systems 16, 21, 22
This approach is based on the pH-dependent release of the drug from the system. In this case the pH differentials between the upper and terminal parts of GI tract are exploited to effectively deliver drugs to the Colon. By combining knowledge of polymers and their solubility at different pH environments, delivery systems have been designed to deliver the drug at the target site.
Eudragit and other enteric coating polymers are widely used to produce acid resistant formulations, and with appropriate control of dissolution time may be reasonably effective in achieving release of drug in the ascending Colon. For Colonic delivery, Eudragit L and S, which are anionic copolymers of methacrylic acid and methyl methacrylate, have been widely used. These polymers are insoluble at low pH but form salts and dissolve above pH 6 and 7 respectively. In vitroevaluation of Eudragit® S and Eudragit® FS was performed and it was found that the, pH-based systems are commercially available as indicated in Table 1.5.20
The proximal Colon is relatively inaccessible. Even large volume enemas will only just reach the transverse Colon. Any substance administered orally has to pass through the hostile environment of the stomach and through the small intestine where it is likely to be digested and absorbed. Protecting the drug from these factors and releasing it at the base of the ascending Colon to optimize Colonic exposure has been the subject of much research.28
Several approaches have been explored to achieve site specific delivery to the Colon. These most commonly include:
i) Utilizing the pH change which occurs on transit from the small intestine to the large intestine
ii) Release of a drug after pre-determined time.
iii) Colon targeting lectins
iv) Utilizing degradation mechanisms of bacteria specific to Colon.
(b) Time dependent systems1
This approach is based on the principle of delaying the release of the drug until it enters into the Colon. Although gastric emptying tends to be highly variable, small intestinal transit time is relatively constant or little bit variation can be observed. This strategy in designing timed-release systems is to resist the acidic environment of the stomach and to undergo a lag time of predetermined span of time, after which release of drug take place. The lag time in this case is the time it requires to transit from the mouth to Colon. The first formulation introduced based on this principle was Pulsincap®.1
An example of such a dosage form would be an impermeable capsule body containing the drug, fitted with a hydrogel plug that is used to deliver the drug after a predetermined time. This dosage form, for example Pulsincap®, releases the drug once the hydrogel plug hydrates and swells in aqueous media and is ejected from the device, thereby allowing the release of the drug from the capsule.21
Another example describes use of a hydrophobic material and surfactant in the tablet coating. The release of drug from the Time Clock® depends mainly on the thickness of the hydrophobic layer and is not dependent on the pH of the GI environment. The rationale behind all time-release delivery systems is valid provided that small intestine transit times remain constant. Changes in GI tract motility can significantly affect time-release drug delivery systems targeting the release of drugs to the Colon.2
(c) Micro flora-activated systems16, 21, 22,24
The microflora of Colon is in the range of 1011 -1012 CFU/mL, consisting mainly of anaerobic bacteria, e.g. Bacteroides, Bifidobacteria, Eubacteria, Clostridia, Enterococci, Enterobacteria and Ruminococcus etc. This vast microflora fulfills its energy needs by fermenting various types of substrates that have been left undigested in the small intestine, e.g. di- and tri-saccharides, polysaccharides etc.
The use of GI microflora as a mechanism of drug release in the Colonic region has been of great interest to researchers in recent times. The majority of bacteria are present in the distal gut although they are distributed throughout the GI tract. Endogenous and exogenous substrates, such as carbohydrates and proteins, escape digestion in the upper GI tract but are metabolised by the enzymes secreted by Colonic bacteria.
Sulphasalazine, a prodrug consisting of the active ingredient mesalazine, was the first bacteria-sensitive delivery system designed to deliver the drug to the Colon. Use of polysaccharides offers an alternative substrate for the bacterial enzymes present in the Colon. Most of the polymers are used in pharmaceutical compositions and are considered generally regarded as safe (GRAS) excipients. Pectin alone and in combination with other polymers has been studied for Colon-specific drug delivery. Pectin, when used alone, was needed in large quantities to control the release of the drug through the core. A coating composition of a mixture of pectin, chitosan and hydroxypropyl methylcellulose was proven to be very efficient as the tablets coated with this composition passed intact through the stomach and small intestine and broke in the Colon.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1.4: Enteric polymers for Colonic drug delivery 21
|
Enteric polymers |
Optimum pH for dissolution |
|
Polyvinyl acetate phthalate (PVAP) (Coateric®) |
5.0 |
|
Cellulose acetate trimellitate (CAT) |
5.5 |
|
Hydroxypropyl methylcellulose phthalate (HPMCP) HP-50 HP-55 and HP-55S |
≥5.0 ≥5.5 |
|
Hydroxypropyl methylcellulose acetate (HPMCAS) *LF Grade *MF Grade *HF Grade |
≥5.5 ≥6.0 ≥6.8 |
|
Methacrylic acid copolymer Type C (Eudragit® L100-55*) Methacrylic acid copolymer dispersion (Eudragit® L30 D-55**) |
≥5.5 |
|
Methacrylic acid copolymer Type C (Eudragit® L100-55*) |
≥6.0 |
|
Cellulose acetate phthalate (CAP) (Aquateric ®) |
6.0 |
|
Methacrylic acid copolymer, Type B (Eudragit® S100*and Eudragit® S12.5) |
≥7.0 |
|
Eudragit® FS 30 D |
≥7.0 |
|
Shellac (MarCOAT 125 and 125 N)
|
7.0 |
Table 1.5: Colonic dosage forms 23
|
S.No. |
Drug |
Polymer |
Dissolution |
Trade Name |
|
1 |
Mesalazine
|
Eudragit L |
6.0 |
Claversal® Mesazal® Calitoflak® |
|
2 |
Mesalazine
|
Eudragit S |
7.0 |
Asacolitin® |
|
3 |
Mesalazine
|
Eudragit L |
6.0 |
Salofalk® |
|
4 |
Mesalazine
|
Ethylcellulose |
- |
Pentasa® |
|
5 |
Sulfasalazine |
Cellulose acetate phthalate |
6.2-6.5 |
Azulfidine® |
|
6 |
Sulfasalazine
|
Eudragit L100-55 |
5.5 |
Colo-pleon® |
|
7 |
Budesonide
|
Eudragit S |
6.0 |
Budenofalk® Entocort® |
(i) Prodrug approach 1, 16
A prodrug is a pharmacologically inactive derivative of a parent molecule that requires some form of transformation in, in vivo to release the active drug at the target site. This approach involves covalent linkage between the drug and its carrier in such a manner that upon oral administration the moiety remains intact in the stomach and small intestine. The type of linkage that is formed between the drug and the carrier would decide the triggering mechanism for the release of the drug in the Colon.
This principle has been exploited commercially to deliver 5-aminosalicyclic acid to the Colon by way of a prodrug carrier. The prodrug sulphasalazine consists of two separate moieties, sulphapyridine and 5-aminosalicylic acid, linked by an azo-bond. The prodrug passes through the upper gut intact, but the azo-bond is cleaved by the host bacteria, liberating the carrier molecule sulphapyridine and the pharmacologically active agent 5-aminosalicylic acid. This concept has led to the development of novel azo-bond based polymers for the purpose of obtaining universal carrier systems.
(ii) Azo-polymeric prodrugs16
Newer approaches are aimed at use of polymers as drug carriers for drug delivery to the Colon. Both synthetic as well as naturally occurring polymers are used for this purpose. Sub synthetic polymers have been used to form polymeric prodrug with azo linkage between the polymer and drug moiety. These have been evaluated for CSDDS, various azo polymers have also been evaluated as coating materials over drug cores. These have been found to be similarly susceptible to cleavage by the azoreducatase in the large bowel. Coating of peptide capsules with polymers cross linked with azoaromatic group has been found to protect drug from digestion in the stomach and small intestine. In the Colon the azo bonds are reduced and the drug is released.
(iii) Polysaccharide based delivery systems13, 16
Use of naturally occurring polysaccharides is attracting lot of attention for drug targeting to the Colon since these polymers of monosaccharides are found in abundance, have wide availability are inexpensive and are available in a variety of a structures with varied properties. They can be easily modified chemically and biochemically and are highly stable, safe, nontoxic, hydrophilic and gel forming and in addition biodegradable. These include naturally occurring polysaccharides obtained from plant (guar gum, inulin), animal (chitosan, chondrotin sulphate), algal (alginates) or microbial (dextran) origin. These are broken down by the Colonic microflora to simple saccharides. So these fall into the category of “generally regarded as safe” (GRAS).
[B] Newly developed Colon-specific drug delivery systems
(a) Intestinal pressure-controlled Colon specific capsules (IPCDCs) 16, 17, 21, 22
Intestinal pressure-controlled Colon specific capsules (PCDCs), relies on the relatively string peristaltic waves in the Colon that led to an increased luminal pressure. It consists of a capsular shaped suppositories coated with a water-insoluble polymer, ethyl cellulose. Once taken orally, PCDCs behave like an ethyl cellulose balloon since the suppository base liquefies at body temperature.
In the upper GI tract, PCDCs are not directly subjected to the luminal pressures since sufficient fluid is present in the stomach and small intestine. Due to the re-absorption of water in the Colon, the viscosity of luminal content increases. As a result, increased intestinal pressures directly affect the system via Colonic peristalsis (high-amplitude propagated contractions). In response to the raised pressure, PCDCs rupture and release the drug load in the Colon.
Based on limited in vivo evaluation, it has been demonstrated that the performance of this system appears to be dependent on the capsule size and the thickness of ethylcellulose coating. The biomagnetic measurement system (BMS) was used to estimate the GI transit characteristics of this system in healthy volunteers. It was found that the capsule arrived at the ascending Colon 4 h and 5 h after oral administration in two subjects, while a model drug, caffeine was first detected in the saliva of the same two subjects 6 h and 5 h following oral administration, respectively. This indicated that PCDCs were able to deliver the drug to the Colon.
(b) CODES™ 16, 17
CODES™ is a unique CDDS technology that was designed to avoid the inherent problems associated with pH- or time-dependent systems. CODES™ is combined approach of pH dependent and microbially triggered CDDS. It has been developed by utilizing a unique mechanism involving lactulose, which acts as a trigger form site specific drug release in the Colon.









