 About Authors:
About Authors:
KAPIL V BIYANI1, DR.P.S. KAWTIKWAR2, N. M. MAHAJAN3
1. M.Pharm, IBSS COLLEGE OF PHARMACY,
BULDHANA ROAD, MALKAPUR (M.S)
2. HOD, SNIOP, PUSAD
3. HOD, IBSS COLLEGE OF PHARMACY,
BULDHANA ROAD, MALKAPUR (M.S)
Introduction1, 2, 3, 4, 5, 6, 7, 8
Capsule is the most versatile of all dosage forms. Capsules are solid dosage forms in which one or more medicinal and inert ingredients are enclosed in a small shell or container usually made of gelatin. There are two types of capsules, “hard”and“soft”. The hard capsule is also called “two pieces” as it consists of two pieces in the form of small cylinders closed at one end, the shorter piece is called the “cap” which fits over the open end of the longer piece, called the “body”. The soft gelatin capsule is also called as “one piece”. Capsules are available in many sizes to provide dosing flexibility. Unpleasant drug tastes and odors can be masked by the tasteless gelatin shell. The administration of liquid and solid drugs enclosed in hard gelatin capsules is one of the most frequently utilized dosage forms.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1220
Advantages of Capsules:
•Capsules mask the taste and odor of unpleasant drugs and can be easily administered.
• They are attractive in appearance
• They are slippery when moist and, hence, easy to swallow with a draught of water.
• As compared to tablets; fewer adjuncts are required.
• The shells are physiologically inert and easily and quickly digested in the gastrointestinal tract.
• They are economical
• They are easy to handle and carry.
• The shells can be opacified (with titanium dioxide) or colored, to give protection from light.
Disadvantages of Capsules
• The drugs which are hygroscopic absorb water from the capsule shell making it brittle and hence are not suitable for filling into capsules.
• The concentrated solutions which require previous dilution are unsuitable for capsules because if administered as such lead to irritation of stomach.
Soft gels – a brief review 9, 10, and 11
Soft gel (a soft gelatin capsule) is a solid capsule (outer shell) surrounding a liquid or semi-solid center (inner fill) active ingredient can be incorporated into the outer shell, the inner fill, or both. The formulation of drugs into soft gelatin capsules has gained popularity throughout the past decade due to the many advantages of this dosage form. The bioavailability of hydrophobic drugs can be significantly increased when formulated into soft gelatin capsules. Many problems associated with tableting, including poor compaction and lack of content or weight uniformity, can be eliminated when a drug is incorporated into this dosage form. Improved stability of drugs that are highly susceptible to oxidation can be achieved when formulated into a soft gelatin capsule.
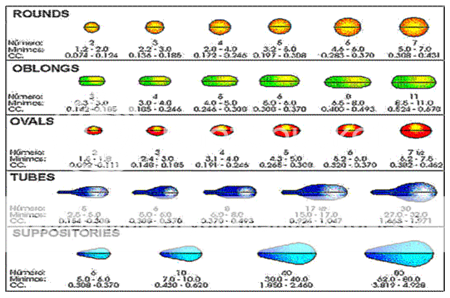
Gelatin soft capsules are made from gelatin and water but with the addition of a polyhydic alcohol, such as glycerol or sorbitol, to make them flexible. Sorbitol is less hygroscopic than glycerol. They usually contain a preservative, such as beta-naphthol. They are available in variety of shapes and sizes.
• Spherical – 0.05 -5 ml
• Ovoid – 0.05 - 7 ml
• Cylindrical – 0.15- 25 ml
• Tubes – 0.5 - 0 ml
• Pear shaped – 0.3 - 5ml
-Fig.1-
They are most suitable for liquids and semisolids and are widely used, in spherical and ovoid forms for vitamin preparations such as cod liver oil, vitamins A and D and multiple vitamins.
Solid dosage forms containing drugs that are susceptible to degradation in the stomach due to the acidic environment or gastric enzymes have been stabilized with an enteric film coating. A decrease in gastric irritation caused by drugs, such as aspirin, can also be achieved by enterically coating the solid dosage form In addition, enteric coatings can be used to target drug release in the small intestine.6 Enteric coating of soft gelatin capsules combines the advantages of the delivery system with the protective properties of the film coating. However, the physicochemical properties of the gelatin and capsule shell present significant challenges to the pharmaceutical scientist when film coating soft gelatin capsules with enteric polymers.
Soft capsules are a single-unit solid dosage form, consisting of a liquid or semi-solid fill enveloped by a one-piece sealed elastic outer shell. The amount of drug or extract together with adjuvant is enclosed within a globular, oval or other shape of a soft shell. Soft gelatin capsules (softgel) offer the possibility of delivering a liquid in a solid oral dosage form. The softgel can contain the active ingredient in solution, suspension or emulsion, which will inherently lead to better absorption of the active ingredient as compared with delivery in a tablet or as a powder.
Highly developed soft capsules are currently being applied widely in the pharmaceutical, chemical, food and cosmetic industry. This is attributed to the features of softgels like desirable aesthetic properties and ‘swallowability’, safety enclosure, precise contents, and pleasing appearance. This has enabled their use as an effective delivery system for hydrophobic drugs, low melting-point drugs, easy-oxidized drugs, and a variety of health care oil. Since the introduction of Soft Capsule Making Machine in the 1970s, formulations have continually become more popular with rapid developments in recent years. This could be illustrated by emergency of a more than 560 sets of Soft Capsule Making Machine with transfer mode having a production rate of up to 60 billion pills/year (i.e.more than 3600 kinds of drugs) in the world. Up to now, there are more than 30 manufacturers producing more than 40 kinds of soft capsules by using over 60 sets of advanced machines. Softgels’ ability to enhance bioavailability not only makes them the preferred dosage form for new chemical entities with poor oral bioavailability, they can also be used for reformulation of existing drugs, with the purpose of life-cycle extension.
The softgel delivery system is a unitary package, formed with gelatin outer layers, which contain between them the active ingredients in solution, suspension or paste form. Hydrophobic drugs do not dissolve readily in water, gastric or intestinal fluid. When they are compounded in solid dosage forms, their dissolution rate is usually low and absorption varies resulting in poor bioavailability. Bioavailability of these drugs can be improved in the presence of fatty acids e.g. mono or diglycerides. Fatty acids do solubilize hydrophobic drugs in the gut and enable more rapid absorption. The softgel delivers drugs in solution and yet offers solid dosage form. These hydrophobic drugs are dissolved in a hydrophilic solvent, which, when crushed or chewed, releases the drug immediately to produce a solution of the drug in gastric juice ready for absorption from the gastrointestinal tract into the blood stream. This results in rapid onset of desired therapeutic effects6. For example, Ibuprofen soft gel gives rise to a shorter time to peak plasma concentration and greater peak plasma concentration compared to a marketed tablet formulation. Cyclosporine does give therapeutic blood levels which are not achievable from tablet form. Similarly oral hypoglycemic glipizide in softgel is also known to have better bioavailability results compared with tablet form. Softgel delivery systems can also incorporate phospholipids or polymers or natural gums to entrap the drug active in the gelatin layer with an outer coating to give desired delayed/control release effects.
The designs for a specific soft gelatin capsule formulation involve appropriate selection of the shell and fill composition. This is followed by optimization of the two to allow for efficient production of a chemically and physically stable product with the desired biopharmaceutical properties. The shell of a soft gelatin capsule is composed of gelatin, a plasticizer or a combination of plasticizers and water. In addition, it may contain preservatives, coloring and opacifying agents, flavorings and sweeteners, possibly sugars to impart chewable characteristics to the shell, gastroresistant substances and in special cases even active compounds8.The formulation of the fill is individually developed to fulfill the requirements for optimum therapeutic action.This entails optimizing the chemical stability of the active compound to improve bioavailability. Emphasis is also put on efficient and safe filling process in order to achieve a physically stable capsule product.
Improved drug absorption is the primary reason for selection of soft gel as a dosage form. Nevertheless as a drug delivery system for oral administration of therapeutic agent several advantages as well as disadvantages have been reported with soft gelatin capsules.
Advantages of soft gel capsules:
1. Ease of use - easy to swallow, no taste, unit dose delivery, temper proof.
2. Versatile
i. Accommodates a wide variety of compounds filled as a semisolid, liquid, gel or paste.
ii. Wide variety of colors, shapes and sizes
iii. Immediate or delayed drug delivery-can be used to improve bioavailability by delivering drug in solution or other absorption enhancing media.
Disadvantages of soft gel capsules:
1. Requires special manufacturing equipment
2. Stability concerns with highly water soluble compounds, and compounds susceptible to hydrolysis
3. Limited choices of excipients/carriers compatible with the gelatin
Content of a softgel capsule is a liquid, or a combination of miscible liquids, a solution of a solid(s) in a liquid(s) or a suspension of a solid(s) in a liquid(s).
Liquids are an essential part of the capsule content. Only those liquids that are both water miscible and volatile cannot be included as major constituents of the capsule content since they can migrate into the hydrophilic gelatin shell and volatilize from its surface. Water, ethyl alcohol and emulsions fall into this category.The softgel dosage form has been around from many years. The earliest soft gels date back to the 19th century. Since then, many improvements have been made with respect to the production of these soft capsules. Softgel manufacturing still requires special skills and equipment that less than a handful of companies can offer to pharmaceutical clients. Notwithstanding the progress that has been made in softgel manufacturing, the softgel as a dosage form has remained largely unchanged over the years. As a result, patent protection on the technology was lost, which is a disadvantage in the era of pharmaceutical life-cycle management. For that reason, Banner has developed new softgel variants that not only offer specific benefits over the standard softgel, but also provide additional patent protection to the compounds they deliver. Soft gelatin capsules (soft gels) offer the possibility of delivering a liquid in a solid oral dosage form. The softgel can therefore contain the active ingredient in solution, suspension or emulsion, which will inherently lead to better absorption of the active ingredient as compared with delivery in a tablet or as a powder. Soft gels are therefore the ideal solution –and sometimes the only solution – for delivery of compounds with poor oral bioavailability. Other properties that make soft gels a useful and frequently applied dosage form include their aesthetic properties and ‘swallowability’, their tamper-resistance, their protection of the active ingredient from light and oxidation, their taste- masking of ingredients and their masking of unpleasant odours of ingredients
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Manufacturing of soft gels:
ENCAPSULATION PROCESS:
Manufacturing soft gelatin capsules implicates the use of sophisticated technology. The rotary type softgel encapsulation process offers accuracy of dosage and higher production capacity. Before encapsulation process begins, Gelatin mass for out shell and medicine for the capsule fill are prepared. The Gelatin powder is mixed with water and glycerin, heated and stirred under vacuum. The outer layer of this special stainless steel vessel is steam- jacketed. Any required flavors or colors are added using a turbine mixer to molten gelatin and transferred to mobile vessels. The gelatin mass is kept in a steam-jacketed storage vessel at a constant temperature. The medicine fill is prepared using standard procedures used in pharmaceutical liquid, paste or suspension manufacturing. The encapsulation process begins when molten gel is pumped to the machine and two thin ribbons of gel are formed on either side of machine. These ribbons then pass over a series of rollers and over a set of die that determine the size and shapes of capsules. The medicine fill is fed from its container to a positive displacement pump, which accurately doses the fill and injects it between two gelatin ribbons prior to sealing them together through the application of heat and pressure.
The capsules formed at this stage are incredibly flexible due to water in gel mass. To remove excess water capsules pass through a conveyer into tumble dryers where about 25% of water is removed. The capsules are then placed on trays which are stacked and transferred into drying rooms where dry air is forced over capsules to remove any excess moisture. The moisture is measured at regular intervals, when the moisture is limited to approx. 8% the drying process is complete and capsules are ready for packaging.
PRODUCTION PROCESS:
The manufacturing of softgel delivery systems is carried out in a high productivity rotator die machine and capsules are dried using an advanced Tumble drier, offering:
• Dosage precision and accuracy.
• Automation.
• Easy cleaning and sanitation.
• High productivity.
• Product variety.
• Encapsulation in absence of oxygen and/or light.
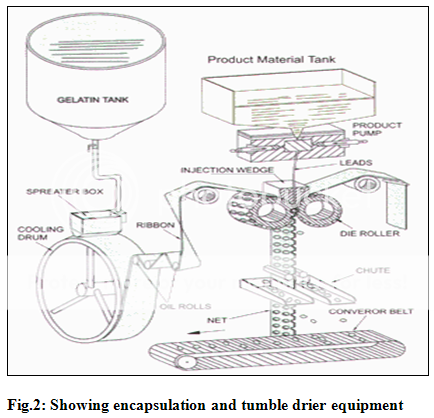
It is also possible to manufacture round seamless capsules (pearls) using a unique technology that allows manufacturing using the physical properties of superficial tension.
The productivity increases or diminishes upon considering the following variables:
- Asset to encapsulate (density, consistency, etc.)
- Capsule size.
- Capsule shape.
Dissolution of soft gelatin capsules:
The bioavailability of the bio -actives in the soft gel depends on the dissolution of both its shell and fill. Dissolution of a chemical compound in the aqueous environment of the gastrointestinal tract is often the rate-limiting step in its absorption. If a substance, such as oil, is insoluble in the acidic solution of the gastrointestinal tract, then its dissolution can be slow. However, administered in a vehicle inwhich it is soluble, then theabsorption process may be enhanced. Polyethylene glycols, cyclodextrins, carboxymethylcellulose, and emulsifiers have been used to enhance solubility of substances in water. Dissolution problems of the soft gel shell are less common; they may become apparent upon aging, which are attributed to the cross-linking of gelatin. The cross-linking causes the formation of a swollen, tough, rubbery, and water-insoluble material. High humidity causes the capsules to become soft, tacky, and bloated and may increase the likelihood of moisture migration from the shell into the fill material. Such a transfer can cause chemical, physical and dissolution instability.
CANDIDATES FOR SOFTGEL DELIVERY SYSTEM:
A number of compounds can be formulated to deliver faster onset of effect with lower dosage and lower side effects. There are at present a number of compounds in early phase of development that could benefit from softgel formulation to give faster absorption, improved and uniform bio availability. Moreover there are a number of poorly-soluble pharmaceutical compounds offering a huge market potential when delivered in softgels. Scherasol from RP Scherer Ltd and BritHealth's Fast Absorption Systems (B-FAS) offer opportunities for these pharmaceutical compounds for faster and uniform absorption.
According to Technology Catalysts International (TCI), there are at least 83 drugs with World-wide sales exceeding US$100 million that are insoluble or poorly soluble in water, and have total combined sales of approximately US$45 billion. Of these candidates, TCI has identified 26 drugs which are most suitable for reformulation in softgel delivery system. If these lead compounds are coupled with novel delivery systems, TCI estimates that the reformulated products will generate an additional US$8 billion in revenues.
The compounds are:
Antihistamines -clemastine, chlorpheniramine, dexchlorpheniramine, astemizole, loratadine, decongestants.
Analgesics- ibuprofen, paracetamol, ketoprofen, naproxen and combinations
Antidepressants- fluoxetine (Prozac), buspirone (Buspar), Phyto compounds
Antinauseants- compazine, chlorpromazine, perphenazine, phyto compounds
Anorexiants- dextroamphetamine, phentermine, mazindol
Biological compounds- bromocryptine, Apo morphine, selegiline, amitriptyline, dopamine precursors, serotonin precursors
Cardiovascular compounds- nitroglycerin, ACE inhibitors, calcium antagonists, beta blockers
Decongestants- dextromethorphan, pseudoephedrine, phenylpropanolamine
Peptides/Proteins and other biopharmaceuticals- cyclosporine, insulin, calcitonin
Sedatives- barbiturates, benzodiazepines
Steroids- testosterone, estradiol, progesterone and combinations
Sleeping Drugs- temazepam, diphenhydramine, zolpidem, triazolam, nitrazepam
Softgel delivery systems also offer opportunities for many new chemical entities including peptides/other biopharmaceuticals and other pharmaceuticals those requiring reformulation due to bio availability concerns/patent extension.
Soft gel reformulation for life cycle management:
Soft gelatin capsules (softgels) offer the possibility of delivering a liquid in a solid oral dosage form. Thesoftgel can therefore contain the active ingredient in solution, suspension or emulsion, which will inherently lead to better absorption of the active ingredient as compared with delivery in a tablet or as a powder. Softgels are therefore the ideal solution –and sometimes the only solution – for delivery of compounds with poor oral bioavailability. Other properties that make softgels a useful and frequently applied dosage form include their aesthetic properties and ‘swallow ability’, their tamper resistance, their protection of the active ingredient from light and oxidation, their taste-masking of ingredients and their masking of unpleasant odors of ingredients.
Softgels’ ability to enhance bioavailability not only makes them the preferred dosage form for new chemical entities with poor oral bioavailability, they can also be used for reformulation of existing drugs, with the purpose of life-cycle extension. Two of such applications are briefly described here:
1. Life-cycle lengthening through new therapeutic indications:
Marketed compounds intended for use in new indications need differentiation from the existing brand in order to discourage generic competition. A method that is underused to prevent off-label use of generics is reformulating the compound with the purpose of changing the active dose level. Compounds that are currently being delivered in a tablet or a capsule could be reformulated in a softgel so as to improve their bioavailability. The compound would then exert the same therapeutic effect at a lower dose level. As a result, the reformulated drug can be promoted-
• With a new brand-name;
• For a new indication;
• In a new, more appealing dosage form; and
• At a dose level that is not easily copied by generic versions of the original dosage form.
With this metamorphosis, the chemical entity can start a new period of its life-cycle.
2. Life-cycle lengthening through product enhancement and faster onset of action:
Pharmaceutical compounds delivered in tablets or regular two-piece hard shell capsules first need to dissolve before they can be absorbed. When delivered in a softgel, the active ingredient – being solubilised already – will be more readily available for absorption and therefore may reach its target receptor more quickly. This can result in a more rapid onset of therapeutic effect. This has been demonstrated clearly with ibuprofen softgels, which give a significantly faster onset of pain relief than analgesic tablets.
Ideal characteristics of soft capsules:
- To optimize the chemical stability of the active compound.
- To improve bioavailability of the active compound
- To allow for an efficient and safe filling process
- To achieve a physically stable capsule product.
- Final product stability is related to shell compatibility
New Soft gel Variants:
The following new dosage forms have been developed:
1. Enteric Soft gel:
In contrast to existing enteric dosage forms, Banner’s new enteric softgel is not coated. The enteric features of the dosage form reside in the shell itself. The result is a clear enteric dosage form with the exact same appeal and patient benefits that the standard softgel offers. Banner’s enteric softgel meets all Pharmacopoeial (American, European, Japanese and British) standards for enteric delivery. Banner’s enteric softgel technology is unique in that it offers a one-step process to manufacture enteric softgels. Traditionally, enteric soft gels were prepared by coating with enteric polymers using traditional coating technology. Coating has its own disadvantages such as unsuccessful adhesion of the enteric polymer onto the soft gelatin shell due to the shell’s inherent flexible nature. This can lead to chipping and peeling of the coat. Enteric coating also results in a hazy and opaque appearance of the capsule and is an additional step of manufacturing.
Advantages of the enteric soft gel over other enteric dosage forms can be summarized as follows:
• Enteric soft gel technology provides enteric properties more consistently than other products because the enteric system is built into the gelatin shell, not just as a coating on top;
• Clear, transparent dosage form, as opposed to coated enteric dosage forms;
• offers the exact same advantages as standard soft gels, including improved ‘swallow ability’, taste-masking and protection against light or oxygen degradation; and
• No leaking problems, as opposed to regular two-piece hard shell capsules.
Candidates for enteric delivery include:
• Compounds those are unstable in gastric acid,
For Example: proton pumps inhibitors, certain antibiotics, triptans and dideoxyinosine (ddI);
• Compounds that is irritating or damaging to the gastric mucosa, for example bisphosphonates, non-steroidal anti-inflammatory drugs, certain antibiotics and carbamazepine;
• compounds targeted at the small intestine, e.g. drugs for the treatment of Crohn’s disease or other intestinal disorders, and drugs that are preferentially being absorbed in the small intestine; and
• Compounds that may cause belching, regurgitation or other gastrogenic discomfort.
Banner has comprehensively designed and optimized the new enteric technology to suit a wide variety of products. We have applied the new technology on different drugs and have generated accelerated stability data. Banner is currently working on an existing drug for scale-up purposes. For this compound, the enteric softgel formulation has successfully passed accelerated stability testing as well.
2. Controlled Release Soft gel:
Banner’s scientists have developed a controlled release technology that is able to achieve a large variety of release patterns. The controlled release soft gel can be applied to a wide range of active molecules. Banner’s controlled release softgel technology uses a lipid matrix in a standard softgel shell. Depending on the physicochemical properties of the active molecule, an emulsion or a suspension is chosen as a matrix. By applying these, or combinations of these, almost any release profile can be engineered simply by varying the formulation. The result is an oral dosage form offering controlled release of the active moiety, combined with all the benefits that the soft gel dosage form offers.
Its release properties, combined with the advantages of a soft gel, make the CR-soft gel a preferred form for those insoluble compounds that require enhanced absorption as well as a prolonged and controlled release.
3. Chewable Soft gels:
The chewable gelatin dosage form offers excellent mouth feel and chewing experience as compared with other chewable dosage forms. Chewable soft gels are particularly suitable for pediatric populations, where swallowing whole tablets or capsules is often a problem and chewable tablets are often rejected. Consumer preference testing with Banner’s new chewable gels showed that three out of four parents would buy this product for their children (TragonResearch, data on file). In the adult population, chewable gels are convenient because they can be taken easily on the run, without the need for water.Lipid-coating of the active ingredient has been used and tested as a means to mask the taste of bitter active ingredients. This approach has resulted in a highly acceptable end-product. Other taste-masking technologies can be combined with the chewable softgel.

Fig 3: -CHEWABLE SOFT GEL-
1. Gelatin-free Soft Capsule:
Gelatin-free soft capsules are made from vegetable ingredients. They have all the advantages of standard soft gels, but do not contain gelatin. Gelatin-free soft capsules are particularly suitable for vegetarians or other populations that prefer non-animal products.
2. Soflet ®Gel caps
Soflet® Gel caps represent a patented technology whereby tablets are enrobed with gelatin. Soflet® Gelcaps are a dosage form preferred by consumers because of the ease of swallowing as well as the taste and odour-masking properties imparted by the gelatin coating. The unique, patented manufacturing process of Soflet® Gel caps results in a single or two-toned colour dosage form that can be imprinted upon. These features offer distinctive opportunities for product branding. Soflet® Gel cap technology is therefore widely used in over the-counter products, both branded and private label. In addition, Soflet® Gel caps are ideal for clinical trial blinding. Banner now also has the capability of manufacturing gelatin-free Soflet® Gel caps.
3. Sustained release capsules :
The traditional method of taking a dose three or four times a day leads to periods of excess and deficiency in blood concentration of the medicament. One way of correcting this and, at the same time, reducing the number of doses per day, is to administer a capsule containing numerous coated pellets that release the drug successively over a long period. The finely powdered drug is first converted into pellets, usually by attaching it to sugar granules with an adhesive. The pellets are then treated with protective coatings that delay release of the drug, each batch receiving a different thickness. The batches are mixed thoroughly and suitable doses are filled into capsules. For example, a mixture might contain 30 percent of uncoated pellets, for immediate release of drug, 30 percent each of coated pellets that release at 4 hours and 8 hours, and 10 percent of neutral pellets, used solely to fill the capsule. Each batch may be colored differently to simplify identification and facilitate control of mixing.
4. Liquid filled hard gelatin capsules :
It is generally accepted that many of today’s NCE’s (New Chemical Entities) are poorly water soluble and the classical methods, such as reduction in particle size are no longer adequate to achieve satisfactory drug adsorption from a solid oral dosage form. One of the most promising strategies to deliver these insoluble compounds is using dissolved systems like using lipids, liquids or semi-solids to formulate new products. Twp piece hard shell capsules are one of the most logical approaches when choosing the best dosage form to deliver these new liquid formulations.
The new technology of packaging liquids in hard gelatin capsules is considered a major breakthrough. It can make a significant contribution to the development of efficacious pharmaceutical products by providing the flexibility to rapidly develop and test in-house formulations when only small quantities of drug substance is available. The process can be scaled-up and also kept in-house similar to the operations of tabletting or powder/pellet filling of hard gelatin capsules.
5. Rectal capsules:
Soft gelatin capsules may be used as substitutes for rectal and vaginal suppositories. Various shapes and sizes are used for this purpose. They are generally wider at one end which is inserted first; the movement of the sphincter muscles forces the capsules forward into the rectum. Liquids or solids can be filled into rectal capsules but the base in which the medicaments have been incorporated must be non-toxic, non-irritant and compatible with the capsule shell
6. Capsules for packing of ophthalmic ointments:
It is very important that the ophthalmic ointments should be sterile and free from irritant effect. Therefore they must be packed in such a manner that the product remains sterile until whole of it is used up. The best method to keep the preparation free from contamination during use is to pack it in single dose containers. Now a days soft gelatin capsules are very commonly used for filling ophthalmic ointments. These capsules are meant for single application to the eye. Just before application, the capsule is punctured with a sterile needle, the contents instilled into the eye and the shell discarded.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
COATING OF SOFT GEL CAPSULES 1, 2, 3,4,5,6
Objectives -
- Coatings have been applied extemporaneously to enhance appearance and conceal taste, as well as to prevent release of the medication in the stomach (enteric coated products).
- Capsules can be coated to delay the release of the active drug until it reaches a selected portion of the gastrointestinal tract. Most coating of capsules requires considerable formulation skill and quality control equipment found in manufacturing facilities.
- Soft gelatin capsules may be post-treated after production or coated to improve product stability, to modify the dissolution rate and to enable enteric capsules to be produced.
- Several patents have been filed describing the use of protective coatings to overcome the stability problems of soft capsules arising from the hygroscopic nature and heat sensitivity of the soft capsule shell.
- Patent line extension.
MATERIALS USED:12 and 13
Materials found suitable include stearic acid, shellac, casein, cellulose acetate phthalate and natural and synthetic waxes; the basis of their use is their acid insolubility but alkaline solubility. Many of the newer coating materials are time: erosion-dependent rather than acid: base-dependent, i.e. they erode over time on exposure to gastrointestinal contents rather than over a pH gradient. There are, in addition, a number of newer materials with predictable pH solubility profiles.
However, most of these attempts have failed in practice, since coating of soft capsules is not an easy task. The low surface, roughness of soft capsule shells and the intrinsic insolubility of the shell components in organic solvents mean that coatings applied as an organic solution usually do not adhere properly to the capsules, resulting in onion-like coatings, layers peeling off immediately after drying or on storage. Aqueous coatings, on the other hand, may result in capsule swelling, softening and/or sticking together, since water is acting as a plasticizer for the gelatin capsule shells. To balance the two extremes, emulsion-based formulations or solutions in a mixture of water and alcohol have been recommended (Osterwaldet al., 1982). The technological approach of choice for soft capsules to be coated is using the fluidisedbed air-suspension technique.
Capsules with modified dissolution characteristics, such as gastro resistant enteric soft gelatin capsules, have been described in the scientific and patent literature and can be achieved by adding
Ø gastro resistant enteric-soluble polymers to the gelatin mass prior to capsule formation, or
Ø by aldehyde post-treatment or
Ø enteric coating of the dried capsules.
All three attempts have their specific difficulties. For soft gelatin capsules produced by the rotary die process, the last two approaches are in practical use.Aldehyde post-treatment of soft gelatin capsules has been known for many years as a popular means to reduce their dissolution rate, i.e. the capsules take a long time to dissolve and have left the stomach before this occurs. Formaldehyde has been described to cross-link effectively soft capsules to render them gastroresistant.Since safety questions have been raised about the presence of trace amounts of formaldehyde in foods and pharmaceuticals, the use of aldehydes without health concerns such as aldoses have been claimed in a patent (Fischer, 1986) and are actually used. The major disadvantage of any aldehyde treatment of soft gelatin capsules is that cross-linking can continue on storage. Alternatively, soft gelatin capsules may be coated with a gastroresistant, enteric-soluble polymer.
Owing to the aforementioned difficulties associated with organic and aqueous soft capsule coating, a formulation and physical properties of soft capsulesprotective subcoat is usually applied as San alcoholic solution prior to the application of the gastroresistant, enteric polymer layer.
Enteric-coated capsules:
Enteric-coated capsules resist disintegration in the stomach but break up in the intestine. They have largely been superseded by enteric-coated tablets. Types of coating used commercially include cellulose acetate phthalate and mixtures of waxes and fatty acids and/or their esters. Enteric coating may be given to following categories of drugs:
• For substances that irritate the gastric mucosa or are destroyed by the gastric juice, and for medicaments, such as amoebicides and anthelmintics that are intended to act in the intestine.
• Which interfere with digestion e.g. tannins, silver nitrate and other salts of heavy metals.
• Which are required to produce delayed action of the drug.
In general, the application of a coating requires skill and additional equipment. A general coating can be applied but should probably only be used in medications that would not be of a critical nature. In many cases, experience must be developed for specific formulations depending upon the requests of the physicians and the needs of the individual patients.
Several coating methods may be used and are described as follows:
Methods for enteric coating of capsules:1, 2, 3,4,5,6
* Beaker-flask coating - Place a very small quantity of the coating material in the flask and gently heat until it has melted. Add a few capsules, remove from the heat and rotate the flask to start application of the coating. Periodically add a few more drops of melted coating material with continued rotation. The addition of very small quantities is all that is required to keep the capsules from sticking together and clumping
* Dipping - Heat the coating material in a beaker at the lowest feasible temperature. Individual capsules can be dipped using tweezers, allowing the coating to cool and repeating the process until a sufficient layer has been developed.
* Spraying - An alcoholic or ethereal solution of the coating material is prepared and placed in a small sprayer (a model airplane paint sprayer works well). The capsules are placed on a screen in a well-ventilated area. The solution of coating material is applied in very thin coats with sufficient time allowed for drying between coats (A hair dryer may be used cautiously for this step). The process is repeated until a sufficient layer has been developed.
ENTERIC POLYMERS USED FOR FILM COATING OF SOLID DOSAGE FORMS:14 to 36
Enteric polymers currently used to coat pharmaceutical dosage forms include cellulose, vinyl, and acrylic derivatives. These polymers exhibit resistance to gastric fluids yet are readily soluble or permeable in intestinal fluid. Enteric polymeric materials are primarily weak acids containing acidic functional groups, which are capable of ionization at elevated pH. In the low pH of the stomach, the enteric polymers are unionized, and therefore, insoluble. As the pH increases in the intestinal tract, these functional groups ionize, and the polymer becomes soluble in the intestinal fluids. Thus, an enteric polymeric film coating allows the coated solid to pass intact through the stomach to the small intestine, where the drug is then released for absorption through the intestinal mucosa into the human body where it can exert its pharmacologic effects.
Ø Cellulose acetate phthalate:
Cellulose acetate phthalate (CAP) was synthesized in 1940 by Hiatt and was one of the first polymers used for its enteric properties. The CAP polymer exhibits rapid dissolution at a pH greater than 6 and is relatively permeable to moisture and gastric juices. Due to its high moisture permeability, CAP is susceptible to hydrolytic decomposition. Phthalic and acetic acid molecules may hydrolyze during storage and significantly compromise the degree of enteric protection that the film coating provides. The addition of a plasticizing agent has been shown to improve the water resistance of CAP films. The CAP polymer is commercially available from FMC Biopolymer under the proprietary name Aquacoat® CPD.
Ø Polyvinyl acetate phthalate:
Polyvinyl acetate phthalate (PVAP) is another enteric polymer commonly used to coat solid dosage forms. This polymer is structurally similar to CAP containing the dicarboxylic phthalic acid in a partially esterified form. Faster release of drug components occurs with PVAP because dissolution of this polymer occurs at a pH of approximately 5.0. Due to its lower moisture permeability, PVAP is relatively more stable to hydrolysis than CAP. PVAP is commercially available from colorcon under the proprietary name Sureteric®.
Ø Several derivatives of hydroxypropyl methylcellulose (HPMC):
Hydroxypropyl methylcellulose (HPMC)also exhibit pH dependent solubility. Shin-Etsu Chemical Co, Ltd. esterified HPMC with phthalic anhydride to produce hydroxypropyl methylcellulose phthalate (HPMCP), which rapidly dissolves in the upper intestinal tract. Due to the limited compatibility of HPMCP with several types of plasticizers, hydroxypropyl methylcellulose acetate succinate (HPMCAS) was developed. The presence of ionizable carboxyl groups in the HPMCAS structure cause the polymer to solubilize at high pH (> 5.5 for the LF grade and > 6.8 for the HF grade). This polymer exhibits good compatibility with a variety of plasticizing agents and is commercially available from Shin-Etsu Chemical Co. Ltd. under the proprietary name AQOAT® in a powdered form to be redispersed in water.
Ø Copolymers of methyl methacrylate and ethyl acrylate:
In the mid 1960s, Lehmann and Dreher developed copolymers of methyl methacrylate and ethyl acrylate as ester components with methacrylic acid for use as enteric polymers.These polymers are produced by an emulsion polymerization process and are commercially available in several forms. The dissolution properties of these polymers are dependent on the content of carboxyl groups in the polymer. These acrylic derivatives are commercially available from Degussa Rhm America under the proprietary name Eudragit®. Eudragit L 30 D-55 is an aqueous-based dispersion containing USP/NF methacrylic acid copolymer Type C and exhibits dissolution above pH 5.5. Acryl-Eze®, commercially available through Colorcon, is a relatively new fully formulated acrylic enteric coating system based on spray-dried USP/NF methacrylic acid copolymer. Type C, containing plasticizer(s), pigment(s), and neutralizing agents in a powder form for redispersion in water. Eudragit FS 30 D is an aqueous-based acrylic polymeric dispersion consisting of methacrylic acid, methyl acrylate, and methyl methacrylate. This polymer contains fewer carboxyl groups and thus dissolves at a higher pH (> 6.5).
Ø Coating with Eudragit ® L 30 D-55:
The enteric coating of soft gelatin capsules (SGC) containing ibuprofen in either PEG 400 or Miglyol© was investigated. The effects of two plasticizers, triethyl citrate (TEC) and tributyl citrate (TBC), on the physical and enteric properties of SGC coated with Eudragit ® L 30 D-55 were studied. The water soluble plasticizer TEC was found to be a good plasticizing agent for the Eudragit® L 30 D-55 irrespective of the fill liquid, while the TBC provided satisfactory results only for capsules containing the hydrophobic fill liquid, Miglyol ®. The combination of TEC and TBC provided effective plasticization for the acrylic coating regardless of the fill liquid. A subcoat of HPMC showed no effect on the enteric protection of either Miglyol® - and PEG-containing capsules that were stored at room temperature and zero percent relative humidity. The moisture content of the gelatin shell of the film coated SGC stored at room temperature and at 0 or 96% relative humidity was followed as a function of time. The load strength of the capsules was measured during 3 months of storage using an Instron universal testing apparatus, and the physical-mechanical properties of the capsules were correlated with the moisture content of .As the moisture content of the gelatin decreased, all formulations exhibited an increase in load strength.
CHALLENGES IN ENTERIC COATING SOFT GELATIN CAPSULES:
Challenges encountered during enteric coating of soft gelatin capsules are often attributed to the properties of the gelatin and the dosage form. Soft gelatin capsules generally contain the medicament dissolved or dispersed in oils or hydrophilic liquids (i.e. fill liquid). The inherent flexibility of the soft gelatin capsule is due to the presence of plasticizers and residual moisture in the capsule shell. Thus, the soft gelatin capsule is a more dynamic system than conventional tablets. As shown in Figure 1, atmospheric moisture may permeate into the capsule shell or into the fill liquid. The drug or fill liquid may migrate into the capsule shell, while the plasticizer or residual water in the gelatin shell can potentially migrate into the fill. A volatile component in soft gelatin capsules may escape into the atmosphere. It is these characteristics that must be considered when enteric coating soft gelatin capsules.
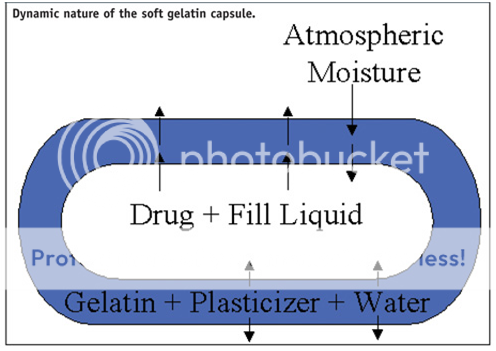
-Fig.4-
Polymeric coatings are generally applied as aqueous-based solutions or dispersions, in which polymer-containing droplets are atomized with air and sprayed onto the substrates. Heat is generally added to the coating equipment to facilitate evaporation of the solvent and film formation. Thus, the processing parameters of spray rate and bed temperature are critical in the coating of soft gelatin capsules. Because gelatin is soluble in water, spraying the aqueous-based polymeric material at a high rate could lead to solubilization of the gelatin and capsule agglomeration. A high bed temperature may result in the evaporation of residual water from the capsule shell, causing the capsule to become brittle.
Soft gelatin capsules must be pre warmed prior to the initiation of the coating process. In the study reported by Felton et al, without this prewarming stage, soft gelatin capsules were very cold to the touch after coating. The outer layers of the film appeared to dry faster than the inner layers, causing bubble formation in the coating. Warming the capsules in the perforated coating pan increased the temperature of the fill liquid to that of the bed temperature, and allowed the coating to dry more uniformly, resulting in a smooth, homogenous film. Pissinati and Oliveira also suggested prewarming of soft gelatin capsules prior to coating in a spouted bed process. As discussed previously, soft gelatin capsules are dynamic systems, with components in the liquid or semi-liquid state capable of diffusional movement. The application of an enteric film coating adds to the complexity of this dosage form. Polymeric films are generally considered brittle, and the addition of a plasticizer is critical to obtain films free of cracks and other defects. The plasticizers in the coating are also capable of diffusional movement into the capsule shell, causing further plasticization and softening of the capsule. Moreover, the plasticizer or residual water in the capsule shell can migrate into the film coating, resulting in further plasticization of the polymer and creating a tacky surface. Finally, the fill material inside the capsule is also capable of permeating through the capsule shell into the polymer film. The migration of these various components, especially during storage, can influence the enteric performance or mechanical strength of the coated capsules.
The application of a barrier film as a subcoat may minimize the potential interaction between the capsule shell and the enteric film coating. Subcoats may also improve polymer adhesion to the capsule shell. Influence of Plasticizer & Fill Liquid on Enteric Performance The addition of a plasticizer in a polymeric film system is generally necessary for the formation of smooth films that are free of cracks and other defects. Plasticizers function by weakening the intermolecular attractions between the polymer chains. These additives have been shown to influence various polymer properties, including the mechanical, adhesive, and drug-release characteristics.13-15 The effects of two plasticizers, triethyl citrate (TEC) and tributyl citrate (TBC), on the physical and enteric properties of soft gelatin capsules coated with Eudragit L 30 D-55 were studied. Size 6 oblong capsules containing ibuprofen dissolved in either hydrophilic polyethylene glycol (PEG 400) or hydrophobic Miglyol® 812 were used in the study. The disintegration results of this study are presented in Table 1.
The water-soluble plasticizer TEC was found to be a good plasticizing agent for the Eudragit L 30 D-55 irrespective of the fill liquid, while the TBC provided satisfactory results only for capsules containing the hydrophobic fill liquid, Miglyol. The combination of TEC and TBC also provided effective plasticization for the acrylic coating regardless of the fill liquid.
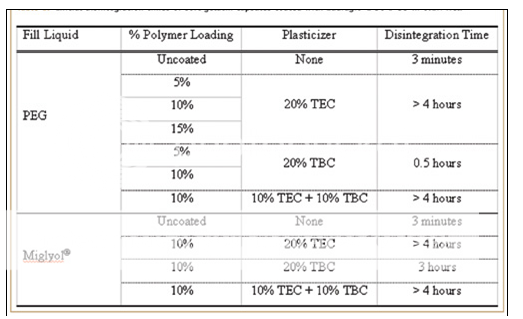
-Table.1-
Initial disintegration times of soft gelatin capsules coated with eudragit L30 D-55 in 0.1 N HCL
Influence of Plasticizer & Fill Liquid on Mechanical Strength The coating of soft gelatin capsules significantly alters the mechanical properties of the dosage form, causing the capsules to become less elastic.
The plasticizing agent incorporated into the film coating, the fill liquid inside the capsule shell, and the amount of polymer coating all have been shown to influence the mechanical strength of the coated soft gelatin capsules. In a study conducted by Felton et al, size 7 round soft gelatin capsules were coated with Eudragit L 30 D-55 and subjected to diametral compression experiments. Influence of Plasticizer & Fill Liquid on Polymer Adhesion Good adhesion between a polymer and the surface of a solid is a major prerequisite for the film coating of pharmaceutical dosage forms. Loss of adhesion may lead to an accumulation of moisture at the film-capsule interface, significantly affecting the stability of drugs susceptible to degradation by hydrolytic mechanisms. Loss of adhesion may also compromise the mechanical protection that the film coating provides to the solid substrate. In addition, experiments on adhesion will be useful to the pharmaceutical scientist during preformulation studies to investigate the relationship between the capsule shell and polymeric film coating formulations.
The two major forces that have been found to affect polymer-tablet adhesion include the strength of the interfacial bond and the internal stresses within the film coating. For pharmaceutical products, hydrogen bond formation is the primary type of interfacial bonding mechanism. Dipole-dipole and dipole-induced dipole interactions also occur, however, to a lesser extent. Factors that affect the type or the number of bonds formed between the polymer and the solid surface will influence film adhesion.The second major factor influencing polymer adhesion is the internal stress within the film. When a polymeric solution or dispersion is applied to a substrate, an internal stress inevitably develops within the film. The total stress within a film is the sum of all the stresses acting on the polymer, including stress due to shrinkage of the film on evaporation of the solvent, thermal stress due to the difference in thermal expansion of the film and the substrate, and volumeric stress due to the change in volume when a substrate swells during storage.
Felton et al used diametral compression experiments as a qualitative measure of polymer-capsule adhesion. The results demonstrated that in most cases, the gelatin shell and the film coating fractured simultaneously, usually at the seam of the capsule, indicating good adhesion between the polymer and the gelatin. In contrast, PEG-containing capsules coated with the TBC-plasticized acrylic polymer showed poor adhesion, as evidenced by the fracture of the film coating followed by the rupture of the capsule shell,The authors attributed the poor adhesion to higher internal stresses within the film coating. In another study conducted by Pissinatti and Oliveira, poor adhesion of an acrylic enteric polymer to soft gelatin capsules, as determined by two-point failure during compression experiments, was also attributed to high internal stress in the film due to inadequate plasticization of the coating.Stability of Enteric Coated Soft Gelatin Capsules Temperature and humidity have been shown to influence stability of nearly all drugs and drug products. 25
Enteric-coated soft gelatin capsules are particularly susceptible to environmental storage conditions due to the dynamic nature of the dosage form. Storage of coated capsules at high humidity may result in agglomeration of the product.The mechanical strength of coated soft gelatin capsules has been shown to be directly proportional to the moisture content of the capsules.10 In another study, the acrylic polymer plasticized with TBC and coated on to soft gelatin capsules containing PEG 400 exhibited an increased adhesion of the polymer to substrate over time when stored at both high temperature and high humidity. The authors attributed these findings to the migration of PEG 400 from the capsule into the polymer film where it functioned to plasticize the polymer. Thus, the internal stresses within the film decreased, allowing for more interfacial interactions and improved adhesion.
Recent updates in Capsule technology 1, 2, 3,4,5,6
A) New products by Capsugel:
1. Capsugel has introduced Oceancaps capsules, these capsules made from all natural fish gelatin derived from farm-raised fish, they have the same characteristics as traditional gelatin capsules, including appearance, machinability, mechanical properties, hygroscopic and oxygen properties, chemical stability, and versatility. Plus, they are odorless and tasteless.
2. Licaps new 000 size capsules are ideal for maximizing liquid dosage with a fill capacity of 1000mg to 1400mg depending on the density of the liquid fill material. This two-piece capsules has been specially designed to be sealed for secure containment of liquids and semi-solids without banding. Available in both gelatin and HPMC (Hydroxypropyl Methylcellulose) capsules they are available in a variety of colors to meet your specific needs.Some of the many benefits of using Licaps capsules include:
- Visually appealing liquid formulation
- Proprietary LEMS® sealing process means no banding and outstanding product integrity
- Available in both gelatin and non-animal capsules and a wide variety of sizes and colors
- Existing products can be reformulated in liquid providing a product line extension
- Strong consumer perception that liquids are fast-acting
- Opportunityfor improved absorption and bioavailability over tablets
- Improved time-to-market versus tablets due to less complex formulation and development processes
- Safer containment for high potency compounds versus tablets
- Improved content uniformity over tablets, particularly at low dosage levels
- Better oxygen barrier than tablets against degradation helps protect the potency of ingredients
- Helps mask taste and unpleasant odors better than softgel capsules
- Outstanding quality – manufactured in accordance with cGMP guidelines with certified quality assurance system for traceability of raw materials
- Offers manufacturing flexibility and can be manufactured in-house or by Capsugel
Unlike soft gels that contain starch and plasticizers, such as glycerin and sorbitol, Licaps liquid-filled capsules allow you to make popular "clean" label product claims such as: Starch-Free, Gluten-Free, No Additives and No Preservatives.
B) New product by Natco Pharma: Hyderabadbased NATCO Pharma Limited has launched LUKATRET - a medicine used in the treatment of a rare form of leukemia.
LUKATRET (Tretinoin - all trans retinoic acid) available in the form of 10 mg. capsules (in a pack of 100 capsules) is used in the treatment of Acute Promyelocytic Leukemia (APL). LUKATRET is a treatment option for remission induction in newly diagnosed, relapsed and / or refractory, chemotherapy non-responsive patients and for patients where anthracycline based chemotherapy is contraindicated. Use of Lukatret results in differentiation and clinical remission.
C) New products by Banner Pharmacaps Inc: Banner Pharmcaps has developed an Enteric Softgel called Entericare, with enteric properties built into the shell matrix of the capsules for delivering very potent (small quantities) as well as drugs that require larger quantities and provide sustained delivery for more than an 8- to 12-hour period.
D) New product by Shionogi Qualicaps : QUALI-V, developed by Shionogi Qualicaps, is the first HPMC capsule developed for eventual use in pharmaceutical products.
E) The Capsugel Liquid-Filled Advancement: Capsugel is meeting growing demand for liquid-filled capsules with our Licaps® capsule, a two-piece hard capsule specially designed for secure containment of liquids and semi-solids.
F) Moisture barrier effect of Kollicoat smartseal 30D: Soft gelatin capsules are known to absorb moisture during storage.This may compromise the active ingredient that are moisture sensitive in the capsules. The moisture protective coating can provide the better stability to these capsules. Uncoated fish oil capsules can absorb moisture 24% of its shell weight when stored in high humidity conditions. The capsules shells stored at ambient conditions normally contains 6-7% moisture. When the capsules are coated with Kollicoat smartseal 30D the moisture absorption tendency is reduced even at 4% coating level.
Quality control of soft gel capsules 1, 2, and 3,4,5,6
Whether capsules are produced on a small scale or large scale all of them are required to pass not only the disintegration test, weight variation test and percentage of medicament test but a visual inspection must be made as they roll off the capsule machine onto a conveyor belt regarding uniformity in shape, size, color and filling. As the capsules moves in front of the inspectors the visibly defective or suspected of being less than the perfect are picked out.
The hard and soft gelatin capsules should be subjected to following tests for their standardization.
1. Shape and size
2. Color
3. Thickness of capsule shell
4. Leaking test for semi-solid and liquid ingredients from soft capsules
5. Disintegration tests
6. Weight variation test
7. Percentage of medicament test
In official books the following quality control tests are recommended for capsules:
Disintegration test:
For performing disintegration test on capsules the tablet disintegration test apparatus is used but the guiding disc may not be used except that the capsules float on top of the water. One capsule is placed in each tube which are then suspended in the beakers to move up and down for 30 minutes, unless otherwise stated in the monograph. The capsules pass the test if no residue of drug or other than fragments of shell remains on No. 10 mesh screen of the tubes.
Weight variation test:
20 capsules are taken at random and weighed. Their average weight is calculated, then each capsule is weighed individually and their weight noted. The capsule passes the test if the weight of individual capsule falls with in 90-110% of the average weight. If this requirement is not met, then the weight of the contents for each individual capsule is determined and compared with the average weight of the contents. The contents from the shells can be removed just by emptying or with the help of small brush. From soft gelatin capsules the contents are removed by squeezing the shells which has been carefully cut. The remainder contents are removed by washing with a suitable solvent. After drying the shells, they are weighed and the content weights of the individual capsules are calculated. The requirements are met if (1) not more than 2 of the differences are greater than 10 % of the average net content and (2) in no case the difference is greater than 25 %.
Content uniformity test:
This test is applicable to all capsules which are meant for oral administration. For this test a sample of the contents is assayed as described in individual monographs and the values calculated which must comply with the prescribed standards.
Capsule stability:
Unprotected soft capsules (i.e., capsules that can breathe) rapidly reach equilibrium with the atmospheric conditions under which they are stored. This inherent characteristic warrants a brief discussion of the effects of temperature and humidity on these products, and points to the necessity of proper storage and packaging conditions and to the necessity of choosing an appropriate retail package The variety of materials capsulated, which may have an effect on the gelatin shell, together with the many gelatin formulations that can be used, makes it imperative that physical standards are established for each product. General statements relative to the effects of temperature and humidity on soft gelatin capsules must be confined to a control capsule that contains mineral oil, with a gelatin shell having a dry glycerin to dry gelatin ratio of about 0.5 to 1 and a water to dry gelatin ratio of 1 to 1, and that is dried to equilibrium with 20 to 30 % RH at 21 to 24 ? C, the physical stability of soft gelatin capsules is associated primarily with the pick-up or loss of water by the capsule shell. If these are prevented by proper packaging, the above control capsule should have satisfactory physical stability at temperature ranging from just above freezing to as high as 60? C, for the unprotected control capsule, low humidities (less than 20 % RH), low temperature (less than 2? C) and high temperatures (greater than 38? C) or combinations of these conditions have only transient effects. the capsule returns to normal when returned to optimum storage conditions. As the humidity is 21 increased, with in a reasonable temperature range, the shell of the unprotected control capsule should pick up moisture in proportion to its glycerin and gelatin content the total moisture content of the capsule shell, at equilibrium with any given relative humidity within a reasonable temperature range, should closely approximate the sum of the moisture content of the glycerin and the gelatin when held separately at the stated conditions effect of temperature and humidity on capsule shell has been illustrated in Table 2 .
Table 2: Effect of Temperature and Humidity on Capsule shell
|
Temperature |
Humidity |
Effect on Capsule shell |
|
21-24°C |
60% |
Capsules become softer, tackier and bloated |
|
Greater than 24°C |
Greater than 45% |
More rapid and pronounced effects – unprotected capsules melt and fuse togethe |
Packaging and storage of capsules:
Capsules should be packed in a well-closed glass or plastic containers and stored in a cool place. These type of containers have advantage over cardboard boxes that they are more convenient to handle and transport and protect the capsules from moisture and dust. To prevent the capsules from rattling a tuft of cotton is placed over and under the capsules in the vials. In vials containing very hygroscopic capsules a packet-containing desiccant like silica gel or anhydrous calcium chloride may be placed to prevent the absorption of excessive moisture by the capsules. Now a days capsules are strip packaged which provide sanitary handling of medicines, ease in counting and identification.
Empty gelatin capsules should be stored at room temperature at constant humidity. High humidity may cause softening of the capsules and low humidity may cause drying and cracking of the capsules. Storage of capsules in glass containers will provide protection not only from extreme humidity but also from dust.
Conclusion:
Interesting advances have recently been made in the area of developing liquid and semi-solid formulation in a soft gelatin capsule to address particular bioperformance issues, namely an increase of bioavailability and decrease of plasma variability by improving solubility and absorption-enhancing techniques. Although the softgel capsules have many advantages, they also face stability problem, mainly for the soft capsules stored for longer than six months. After this time, their soluble products decreased, and the remnants cannot be re-dissolved and/or reabsorbed into the gastrointestinal tract. These problems could be investigated as a research subject.
The soft gel is a versatile oral dosage form that has applications for several drugs, in particular those with poor oral bioavailability. The availability of new softgel variants enteric softgel, controlled release soft gel, chewable soft capsule and gelatin-free soft gel will enhance the application of soft gels in the pharmaceutical marketplace even further, specifically also for drugs that require life-cycle lengthening.
References:
1) Pharmaceutics – The Science of Dosage form Design by M.E. Aulton, 2nd Edition.
2) Remington – The Science and Practice of Pharmacy, 20th Edition, Volume–1.
3) The Theory and Practice of Industrial Pharmacy by Leon Lachman.
4) Encyclopedia of Pharmaceutical Technology, Volume – 2.
5) Textbook of Pharmaceutics by Bentley, 8th Edition.
6) Modern Pharmaceutics by Gilbert S. Bankers, 4th Edition, Dekker series.
7) Ogura T, Furuya Y, Matuura S. HPMC capsules: an alternative to gelatin. Pharm Tech Europe. 1998; 10(11): 32-42.
8) Gelatin. Gelatin Manufacturer’s Institute of America.B Packman,et al., “Solubilized ibuprofen: evaluation of onset, relief, and safety of a novel formulation in the treatment of episodic tension-type headache”, Headache,40 (7), Jul–Aug 2000, pp. 561–7.
9) E V Hersh, et al., “Ibuprofen liquigel for oral surgery pain”, Clin. Ther., 22 (11), Nov 2000, pp. 1,306–8.
10) N Z Olson, et al., “Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain”, J. Clin. Pharmacol. 41 (11), Nov 2001, pp. 1,238–47.
11) G Doyle, et al., “Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain”, J. Clin. Pharmacol., 42 (8), Aug 2002, pp. 912–9.
12) Website –capsugel.com
13) Website –liquidcapsules.com
14) Zaghloul AA, Gurley B, Khan M, Bhagavan H, Reddy I, et al. Bioavailability assessment of oral coenzyme Q10 formulations in dogs. Drug Dev Ind Pharm. 2002; 28:1195-1200.
15) Schettler T, Paris S, Pellett M, Kidner S, Wilkinson D. Comparative pharmacokinetics of two fast-dissolving oral ibuprofen formulations and a regular release ibuprofen tablet in healthy volunteers. Clin Drug Invest. 2001;21:73-78.
16) Seager H. Soft gelatin capsules: a solution to many tableting problems. Pharm Tech. 1985; 9:84-104.
17) Hom FS, Veresh SA, Ebert WR. Soft gelatin capsules, part II: oxygen permeability study of capsule shells. J Pharm Sci. 1975; 64:851-857.
18) Petroski D. Endoscopic comparison of various aspirin preparations: gastric mucosal adaptability to aspirin restudied. Curr Ther Res Clin Exp. 1989;45:945-954.
19) Cole ET, Scott RA, Connor AL, Wilding IR, Petereit H-U, Schminke C, Beckert T, Cade D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm. 2002;231:83-95.
20) Gumowski F, Doelker E, Gurny R. Pharm Tech. 1987;26:27-32.
21) Rowe RC, Sheskey PJ, Weller PJ. Handbook of Pharmaceutical Excipients, Fourth Edition. Chicago: American Pharmaceutical Association; 2003.
22) Lehmann K, Dreher D. Pharm Ind. 1969; 31:319-322.
23) Felton LA, Haase MM, Shah NH, Zhang G, Infeld MH, Malick AW, McGinity JW. Physical and enteric properties of soft gelatin capsules coated with Eudragit® L 30 D-55. Int J Pharm. 1995;113:17-24.
24) Pissinati R, Oliveira P. Enteric coating of soft gelatin capsules by spouted bed: effect of operating conditions on coating efficiency and on product quality. Eur J Pharm Biopharm. 2003;55:313-321.
25) Felton LA, Shah NH, Zhang G, Infeld MH, Malick AW, McGinity JW. Physical-mechanical properties of film-coated soft gelatin capsules. Int J Pharm. 1996; 127:203-211.
26) Gutierrez-Rocca JC, McGinity JW. Influence of water soluble and insoluble plasticizers on the physical and mechanical properties of acrylic resin copolymers. Int J Pharm. 1994; 103:293-301.
27) Felton LA, McGinity JW. Influence of plasticizers on the adhesive properties of an acrylic resin copolymer to hydrophilic and hydrophobic tablet compacts. Int J Pharm. 1997; 154:167-178.
28) Amighi K, Moes A. Influence of plasticizer concentration and storage conditions on the drug release rate from Eudragit RS-30D film coated sustained release theophylline pellets. Eur J Pharm Biopharm. 1996; 42:29-35.
29) Rowe RC. The adhesion of film coatings to tablet surfaces-the effect of some direct compression excipients and lubricants. J Pharm Pharmacol. 1977;129;723-726.
30) Felton LA, McGinity JW. Adhesion of polymeric films to pharmaceutical solids. Eur J Pharm Biopharm. 1999;47:3-14.
31) Okhamafe AO, York P. The adhesion characteristics of some pigmented and unpigmented aqueous-based film coatings applied to aspirin tablets. J Pharm Pharmacol. 1985;37:849-853.
32) Stanley P, Rowe RC, Newton JM. Theoretical considerations of the influence of polymer film coatings on the mechanical strength of tablets. J Pharm Pharmacol. 1981; 33:557-560.
33) Rowe RC. A reappraisal of the equations used to predict the internal stressed in film coatings, applied to tablet substrates. J Pharm Pharmacol. 1983;35:112-113.
34) Felton LA, Friar AL. Enteric coating of gelatin and cellulosic capsules using an aqueous-based acrylic polymer. Pharm Sci. 2002;4:Abstract T3320.
35) Thoma K, Bechtold K. Enteric coated hard gelatin capsules. Capsugel Tech Bull. 1986:1-16.
36) Murthy KS, Enders NA, Mahjour M, Fawzi MB. A comparative evaluation of aqueous enteric polymers in capsule coating. Pharm Tech. 1986;10:36-4
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









