 About Authors:
About Authors:
Tarun Parashar1*, Soniya1, Satyanand Tyagi2, Patel Chirag J3, Rishikesh Gupta4, Ram Narayan Prajapati4
1*Department of Pharmaceutics, Himalayan Institute of Pharmacy and Research, Rajawala, Dehradun, Uttarakhand, India-248002.
2President, Tyagi Pharmacy Association & Scientific Writer (Pharmacy), Chattarpur, New Delhi, India-110074.
3Department of Pharmaceutics, Maharishi Arvind Institute of Pharmacy, Mansarovar, Jaipur, Rajasthan, India-302020.
4Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India-284128.
*parashar89tarun@gmail.com, +91-7838447014/08006939831
ABSTRACT:
Heat shock proteins (HSP) are a group of proteins the expression of which is increased when the cells are exposed to elevated temperatures. HSP are present in cells under normal conditions, but are expressed at high levels when exposed to sudden temperature jump or other stress. HSP stabilize proteins and are involved in the folding of denatured proteins. High temperatures and other stresses, such as altered pH and oxygen deprivation, make it more difficult for proteins to form their proper structures and cause some already structures protein to unfold. Left uncorrected, mis-folded proteins form aggregates that may eventually kill the cell. HSB are induced rapidly at highly levels to deal with this problem. HSP have wide clinical applications, they are not only useful as Cancervaccine adjuvant as well as anticancer therapeutics, and also they are useful in agricultural field. The aim of present article is to provide in depth knowledge about clinical indications of these proteins so called as “Heat Shock Proteins”.
An attempt is also made to focus on functions, characteristics, types, qualities, clinical significance as well as brief description of Heatshock proteins.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1541
INTRODUCTION
Heat shock proteins (HSP) are a class of functionally related proteins involved in the folding and unfolding of other proteins. Their expression is increased when cells are exposed to elevated temperatures or other stress [1]. This increase in expression is transcriptionally regulated. The dramatic up regulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF) [2]. HSPs are found in virtually all living organisms, from bacteria to humans. Heat-shock proteins are named according to their molecular weight. For example, Hsp60, Hsp70 and Hsp90 (the most widely-studied HSPs) refer to families of heat shock proteins on the order of 60, 70, and 90 kilodaltons in size, respectively [3]. The small 8-kilodalton protein ubiquitin, which marks proteins for degradation, also has features of a heat shock protein [4].
It is known that rapid heat hardening can be elicited by a brief exposure of cells to sub-lethal high temperature, which in turn provides protection from subsequent and more severe temperature. In 1962, Ritossa reported that heat and the metabolic uncoupler dinitrophenol induced a characteristic pattern of puffing in the chromosomes of Drosophila [5, 6]. This discovery eventually led to the identification of the heat-shock proteins (HSP) or stress proteins whose expression these puffs represented. Increased synthesis of selected proteins in Drosophila cells following stresses such as heat shock was first reported in 1974 [7]. Beginning in the mid-1980s, investigators recognized that many HSPs function as molecular chaperones and thus play a critical role in protein folding, intracellular trafficking of proteins, and coping with proteins denatured by heat and other stresses. Therefore, the study of stress proteins has undergone explosive growth [8].
[adsense:468x15:2204050025]
FUNCTIONS OF HEAT SHOCK PROTIENS
The functions of heat shock proteins may be listed as follows-:
?Upregulation in Stress
Production of high levels of heat shock proteins can also be triggered by exposure to different kinds of environmental stress conditions, such as infection, inflammation, exercise, exposure of the cell to toxins (ethanol, arsenic, trace metals, and ultraviolet light, among many others), starvation, hypoxia (oxygen deprivation), nitrogen deficiency (in plants), or water deprivation.
As a consequence, the heat shock proteins are also referred to as stress proteins and their upregulation is sometimes described more generally as part of thestress response [9]. The mechanism by which heat-shock (or other environmental stressors) activates the heat shock factor has been determined in bacteria. During heat stress outer membrane proteins (OMPs) do not fold and cannot insert correctly into the outer membrane. They accumulate in the periplasmic space. These OMP's are detected by DegS, an inner membrane protease, that passes the signal through the membrane to the sigmaE transcription factor [10]. However, some studies suggest that an increase in damaged or abnormal proteins brings HSPs into action. Some bacterial heat shock proteins are upregulated via a mechanism involving RNA thermometers such as the FourU thermometer, ROSE element and the Hsp90 cis-regulatory element [11].
? Role as Chaperone
Heat shock proteins function as intra-cellular chaperones for other proteins. They play an important role in protein-protein interactions such as folding and assisting in the establishment of proper protein conformation (shape) and prevention of unwanted protein aggregation. By helping to stabilize partially unfolded proteins, HSPs aid in transporting proteins across membranes within the cell [12, 13]. Some members of the HSP family are expressed at low to moderate levels in all organisms because of their essential role in protein maintenance.
? Housekeeping
Heat-shock proteins also occur under non-stressful conditions, simply "monitoring" the cell's proteins. Some examples of their role as "monitors" are that they carry old proteins to the cell's "recycling bin" (proteasome) and they help newly synthesized proteins fold properly. These activities are part of a cell's own repair system, called the "cellular stress response" or the "heat-shock response".
? Cardiovascular
Heat shock proteins appear to serve a significant cardiovascular role. Hsp90, hsp84, hsp70, hsp27, hsp20, and alpha B crystallin all have been reported as having roles in the cardio vasculature [14]. Hsp90 binds both endothelial nitric oxide synthase and soluble guanylate cyclase, which in turn are involved in vascular relaxation[15]. A downstream kinase of the nitric oxide cell signalling pathway, protein kinase G, phosphorylates a small heat shock protein, hsp20. Hsp20 phosphorylation correlates well with smooth muscle relaxation and is one significant phosphoprotein involved in the process [16].
Hsp20 appears significant in development of the smooth muscle phenotype during development. Hsp20 also serves a significant role in preventing platelet aggregation, cardiac myocyte function and prevention of apoptosis after ischemic injury, and skeletal muscle function and muscle insulin response [17]. Hsp27 is a major phosphoprotein during muscle contraction. Hsp27 functions in smooth muscle migration and appears to serve an integral role [18].
? Immunity
Extracellular and membrane bound heat-shock proteins, especially Hsp70 are involved in binding antigens and presenting them to the immune system [19].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CHARACTERSTIC FEATUTRES OF HEAT SHOCK PROTIENS
HSP are present in cells under normal conditions, but are expressed at high levels when exposed to sudden temperature jump or other stress. HSP stabilize proteins and are involved in the folding of denatured proteins. High temperatures and other stresses, such as altered pH and oxygen deprivation, make it more difficult for proteins to form their proper structures and cause some already structures protein to unfold. Left uncorrected, mis-folded proteins form aggregates that may eventually kill the cell. HSB are induced rapidly at highly levels to deal with this problem. Most heat shock proteins are molecular chaperones (Fig. 1) aid in the transport of proteins throughout the cell’s various compartments [20]. In a proteins unfolded state, the hydrophobic groups will be exposed, allowing hydrophobic interactions with other poly peptide strands and thereby aggregating. Chaperones provide “shelters” (Fig. 2) in which new protein chains can be “incubated” until they have folded properly [21, 22]. The Fig. 3 shows the role of heat-shock proteins and a chaperonin in protein folding.
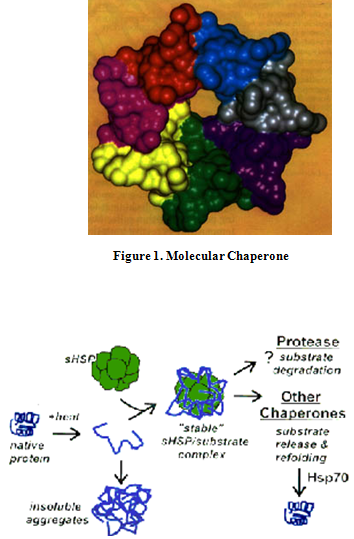
Figure 2. Heat-denatured proteins can either aggregate to form insoluble complexes, or can be prevented from aggregating by binding to sHsps. The denatured protein can then be degraded by proteases in the cell, or refolded by ATP-dependent chaperons like HSP70.
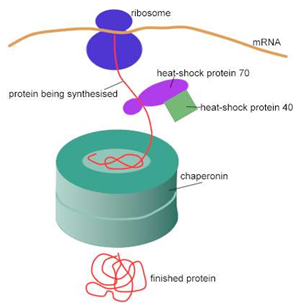
Figure 3. Role of heat-shock proteins and a chaperonin in protein folding.
TYPES AND QUALITIES OF HSPs
Humans, fruit flies and plants all have HSPs very similar in sequence and in structure. HSPs are classified by their molecular weight, size, and in structure. HSPs are classified by their molecular weight, size, structure, and function. They are divided into several families [23], namely:
?HSP 100
? HSP 90
? HSP 70
? HSP 60 (chaperonin)
? Small Heat Shock Proteins/ (alpha)-crystallin proteins
Some Qualities of Heat Shock Proteins and the Reasons, Why Don’t Heat Shock Proteins Denature?
? Better Hydrogen Bonds
? Better Hydrophobic Internal Packing
? Enhanced Secondary Structure
? Helix Dipole Stabilization
CLINICAL SIGNIFICANCE
Heat Shock Factor 1 (HSF1) is a transcription factor that is involved in the upregulation of Hsp70 protein expression[24]. Recently it was discovered that HSF1 is a powerful multifaceted modifier of carcinogenesis. HSF1 knockout mice show significantly decreased incidence of skin tumor after topical application of DMBA (7, 12-dimethylbenzanthracene), a mutagen [25].
APPLICATIONS OF HSPs
* Cancer Vaccine Adjuvant
Given their role in antigen presentation, HSPs are useful as immunologic adjuvants in boosting the response to a vaccine. Furthermore, some researchers speculate that HSPs may be involved in binding protein fragments from dead malignant cells and presenting them to the immune system. Therefore HSPs may be useful for increasing the effectiveness of cancer vaccines [26].
* Anticancer Therapeutics
Intracellular heat shock proteins are highly expressed in cancerous cells and are essential to the survival of these cell types. Hence small molecule inhibitors of HSPs, especiallyHsp90 show promise as anticancer agents [27]. The potent Hsp90 inhibitor 17-AAG is currently in clinical trials for the treatment of several types of cancer [28]. HSPgp96 also shows promise as an anticancer treatment and is currently in clinical trials against non-small cell lung cancer.
* Agricultural
Researchers are also investigating the role of HSPs in conferring stress tolerance to hybridized plants, hoping to address drought and poor soil conditions for farming [29].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MISCELLANEOUS ASPECTS ABOUT HSPS
? Heat shock proteins (HSP) are expressed in response to various biological stresses, including heat, high pressures, and toxic compounds. It is also one of the most abundant cellular proteins found under non-stress conditions.
? Hsp90 is part of a family of proteins known as "chaperones," which are solely dedicated to helping other proteins fold and assume their proper functions.
? The chaperones Hsp70 and Hsp90 together with co-chaperones function to fold proteins in the cytoplasm. Sometimes Hsp70 and Hsp90 function sequentially to fold the same protein.
? WhenHSP90 is compromised the number of morphological changes increases, which lead to formation of inactive or abnormally active polypeptides.
? Changes in protein conformation are involved in some of the most devastating and intractable diseases. “Studies in yeast may help us decipher the fundamental nature of these disorders, including Creutzfeldt-Jakob, Alzheimer’s, Huntington’s, and Parkinson’s disease in humans and mad cow disease in cattle. Several of the protein culprits are being imported into yeast, which allows for the manipulation & study of their folding transitions & testing of therapeutic strategies.
DISCUSSION AND CONCLUSION
The function of a protein is determined by its three-dimensional structure. When excessive heat is applied to proteins, chains of amino acids which are folded into spirals, loops and sheets begin to loose their shapes. When the interior of these proteins gets exposed, proteins can adhere and form globs. This can make them dysfunctional. Protein conformational defects are responsible for a number of pathologies, ranging form Alzheimer’s disease and oncogenic transformation in humans to heat and drought susceptibility in plants. Chaperones protect against denaturization. Heat shock proteins bind to denatured proteins to prevent aggregation. Some HeatShock Proteins, like Hsp104, have the ability to rescue already aggregated proteins. The highly conserved heat shock proteins (HSPs) are constitutively expressed and function as molecular chaperones which facilitate the synthesis and folding of proteins. They also participate in protein assembly, export, turn-over and regulation.
Under stressful conditions such as heat shock, pH shift or hypoxia, increased expression of HSPs protect the cell by stabilizing unfolded proteins, giving the cell time to repair or re-synthesize damaged proteins. HSP90 is a powerful evolutionary mechanism that ensures apparent genetic stability at physiological conditions and at the same time allows the mutations that could rapidly become manifest under stress. HSPs are successfully used as cancer vaccine adjuvant as well as anti cancer therapeutics, they have also found role in agricultural scenario. Recent developments in Hsp90-related pharmacology and the establishment of several clinical methods which utilize the role of chaperones in peptide presentation make this field an exciting area for future clinical studies. Even in our own private life we may take a lesson from chaperones: small, but frequent challenges lead to a modest induction of heat shock proteins, which increases our fitness and (at least in animal studies) prolongs life expectancy.
ACKNOWLEDGEMENT
As a corresponding author, My Self, Tarun Parashar is highly thankful to my Parents and Teachers for their moral support and encouragement. Last but not the least, thanks to Almighty god for showering his blessings best owned on me.
REFERENCES
1. De Maio A, "Heat shock proteins: facts, thoughts, and dreams", Shock (Augusta, Ga.), 1999, 11 (1), 1–12.
2. Wu C, "Heat shock transcription factors: structure and regulation", Annual review of cell and developmental biology, 1995, 11, 441–69.
3. Li Z, Srivastava P, "Heat-shock proteins", Current Protocols in Immunology, 2004, Appendix 1: Appendix 1T.
4. Raboy B, Sharon G, Parag HA, Shochat Y, Kulka RG, "Effect of stress on protein degradation: role of the ubiquitin system", Acta biologica Hungarica, 1991, 42 (1–3), 3–20.
5. Ritossa F, "A new puffing pattern induced by temperature shock and DNP in drosophila", "A new puffing pattern induced by temperature shock and DNP in drosophila", Cellular and Molecular Life Sciences (CMLS), 1962, 18 (12), 571–573.
6. Ritossa F, "Discovery of the heat shock response", Cell Stress Chaperones, 1996, 1 (2), 97–8.
7. Schlesinger MJ, "Heat shock proteins", The Journal of Biological Chemistry, 1990, 265 (21), 12111–12114.
8. en.wikipedia.org/wiki/Heat_shock_protein
9. Santora MG, “Heat shock factors and the control of the stress response”, Biochemical pharmacology, 2000, 59(1), 55-63.
10. Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT, "OM peptide signals initiate the envelope stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain", Cell, 2003, 113 (1), 61–71.
11. Narberhaus F, “Translational control of bacteria heat shock and virulence genes by temperature-sensing mRNAs”, RNA Biol, 2010, 7(1), 84-9.
12. Walter S, Buchner J, Molecular chaperones--cellular machines for protein folding", Angewandte Chemie, 2002, 41(7), 1098-113.
13. Borges JC, Ramos CH, "Protein folding assisted by chaperones", Protein and peptide letters, 2005, 12 (3), 257–61.
14. Benjamin IJ, McMillan DR, “Stress (heat shock) proteins: molecular chaperons in cardiovascular biology and disease, Circulation Research, 1998, 83(2), 117-32.
15. Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, Catravas JD, “Functional significance of hsp90 complexes with NOS and sGC in endothelial Cells”, Clinical Hemorheology and microcirculation, 2007, 37(1-2), 19-35.
16. McLemore EC, Tessier DJ, Thresher J, Komalavilas P, Brophy CM, "Role of the small heat shock proteins in regulating vascular smooth muscle tone", Journal of the American College of Surgeons, 2005, 201 (1), 30–6.
17. Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG, "Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury", Circulation, 2005, 111 (14), 1792–9.
18. Salinthone S, Tyagi M, Gerthoffer WT, "Small Heat Shock Proteins in Smooth Muscle", Pharmacology & therapeutics, 2008, 119 (1), 44–54.
19. Nishikawa M, Takemoto S, Takakura Y, "Heat shock protein derivatives for delivery of antigens to antigen presenting cells", Int J Pharm, 2008, 354 (1–2), 23–7.
20. cs.stedwards.edu/chem/Chemistry/CHEM43/CHEM43/HSP/STRUCTURE.HTML
21. Adhern, Mathews, VanHold. Biochemistry Third Edition. New York: Addison Wesley.
22. antigenics.com/tech/f why.html
23. biologists.com/JCS/110/13/jcs8125.html
24. D Zalmas LP, La Thangue NB, "A transcription cofactor required for the heat-shock response", EMBO Rep, 2008, 9 (7), 662–9.
25. Dai C, Whitesell L, Rogers AB, Lindquist S, "Heat Shock Factor 1 Is a Powerful Multifaceted Modifier of Carcinogenesis", Cell, 2007, 130 (6), 1005–18.
26. Binder RJ, "Heat-shock protein-based vaccines for cancer and infectious disease", Expert Rev Vaccines, 2008, 7 (3), 383–93.
27. Didelot C, Lanneau D, Brunet M, et al, "Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins", Curr. Med. Chem, 2007, 14 (27), 2839–47.
28. Solit DB, Rosen N, "Hsp90: a novel target for cancer therapy", Curr Top Med Chem, 2006, 6 (11), 1205–14.
29. Vinocur B, Altman A, "Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations", Current opinion in biotechnology, 2005, 16 (2), 123–32.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









