 ABOUT AUTHORS
ABOUT AUTHORS
Chaitanya Prasad Meher*2 , M.V.Kumar1, V.Sravanthi1 , K.Ramya1, K.Satyanarayana1
1Maheshwara College of Pharmacy, Chitkul, Isnapur,
Patancheru, Hyderabad-502307 (A.P)
2Assistant professor, Department of pharmaceutical chemistry,
Maheshwara College of Pharmacy, Hyderabad-502307 (Andhra Pradesh)
*chaitanyameher84@gmail.com
ABSTRACT
Neuroscience is a broad, multidisciplinary field concerned with the nervous system, its components, and functional activities, including behavior and consciousness. It relates to nerve cells function and development, how do they communicate, how do brains work, and how have they evolved, Nature of consciousness, and the neural basis for behaviors and for human brain dysfunction. These are among the many questions being answered by contemporary neuroscience. The present review article is concern on the aspect that usually reated to the neurochemistry.
REFERENCE ID: PHARMATUTOR-ART-1719
INTRODUCTION
Neurochemistry is the specific study of neurochemicals, including neurotransmitters and other molecules such as neuro-active drugs that influence neuron function. This principle closely examines the manner in which these neurochemicals influence the network of neural operation. This evolving area of neuroscience offers a neurochemist a micro-macro connection between the analysis of organic compounds active in the nervous system and neural processes such as cortical plasticity, neurogenesis and neural differentiation1. The balance and distribution of chemical messengers (neurotransmitters) of the brain and nervous system. None of the billions of nerve cells, or neurons, in the human brain functions alone. To process information, neurons must form circuits and must communicate with each other rapidly and with great precision. Within a neuron, the electrical impulses that carry information are propagated by rapid changes in membrane potential that arise from the controlled opening and closing of ion channels. These pores in the cell membrane permit the controlled passage of positive or negative ions between the interior and exterior of the cell, and thereby the conduction of electrical impulses along the cell's processes. Additional mechanisms are required at synapses (neuron junctions) to pass signals from one neuron to another. Although a few neurons form electrical synapses, where electrical signals are conducted directly from one neuron to the other through specialized ion channels (gap junctions), most neurons in the mature nervous system communicate via chemical synapses. At chemical synapses, electrical activity in a presynaptic neuron causes the release of a chemical messenger, a neurotransmitter, which diffuses across the narrow synaptic cleft to bind to neurotransmitter receptors on the postsynaptic neuron and elicit changes in the electrical activity of that neuron. After release, all neurotransmitters bind to neurotransmitter receptors and initiate changes in the postsynaptic neuron. It is the biochemical properties of the receptor protein, rather than that of the neurotransmitter itself, that determine the response of the postsynaptic cell. Each neurotransmitter binds to a different receptor, although multiple receptor types exist for several neurotransmitters, with each receptor initiating a different response in the target neuron. Functionally, neurotransmitter receptors fall into two groups, based on the mechanisms by which they alter the electrical activity of a neuron. Ionotropic receptors include an ion channel as part of their structure, and binding of the neurotransmitter results in immediate opening of that ion channel. Metabotropic receptors influence ion channels indirectly through activation of one of several second-messenger pathways. The three second-messenger systems that have been identified so far are similarly organized in that each includes a ligand-binding receptor domain coupled to a transducer that regulates the activity of an effector enzyme. The enzyme produces a second messenger that acts directly on one or more target proteins or activates additional, secondary effector enzymes. In addition to regulating ion channels, second-messenger systems may influence a variety of intracellular processes and elicit long-lasting changes in stimulated neurons.
Once a neurotransmitter has activated its receptors, it must be removed or destroyed rapidly in order to permit transmission of subsequent signals. Some neurotransmitters, regardless of type, simply diffuse from the synaptic cleft. Small-molecule neurotransmitters are also taken back up by presynaptic and postsynaptic neurons and by neighboring cells. One neurotransmitter, acetylcholine (ACh), is broken down rapidly by a membrane-bound enzyme in the region of the synapse. Neuroactive peptides are eliminated only by diffusion from the synaptic cleft and by proteolysis (degradation) by extracellular enzymes; thus they tend to have more sustained effects than small-molecule neurotransmitters. The various aspect of neurochemistry that are closely related are described below.
AMINO ACID NEUROTRANSMITTER- An amino acid neurotransmitter is a chemical substance which is able to transmit a nerve message across a synapse. Neurotransmitters(chemicals) are packaged into vesiclesthat cluster beneath the axon terminalmembrane on the presynaptic side of a synapsein a process called endocytosis. Amino acid neurotransmitter release (exocytosis) is dependent upon calcium Ca2+and is a presynapticresponse. There are inhibitoryamino acids (IAA) or excitatoryamino acids (EAA). Some EAA are L-Glutamate, L-Aspartate, L-Cysteine, and L-Homocysteine.2 These neurotransmitter systems will activate post-synapticcells. Some IAA include GABA, Glycine, β-Alanine, and Taurine. The IAA depressthe activity of post-synaptic cells. 3
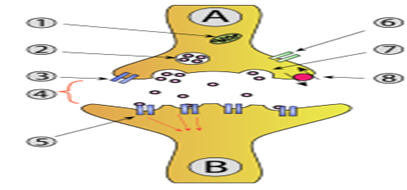
Activity at an axon terminal: Neuron A is transmitting a signal at the axon terminal to neuron B (receiving). Features: 1. Mitochondrion. 2. synaptic vesiclewith neurotransmitters. 3. Autoreceptor. 4. Synapsewith neurotransmitter released (serotonin). 5. Postsynaptic receptors activated by neurotransmitter (induction of a postsynaptic potential). 6. Calcium channel. 7. Exocytosis of a vesicle. 8. Recaptured neurotransmitter
[adsense:468x15:2204050025]
AMYLOID PRECURSOR PROTEIN(APP)- Amyloid precursor protein (APP) is an integral membrane proteinexpressed in many tissuesand concentrated in the synapsesof neurons. Its primary function is not known, though it has been implicated as a regulator of synapse formation,4 neural plasticity5and iron export.6 APP is best known as the precursor molecule whose proteolysisgenerates beta amyloid(Aβ), a 37 to 49 amino acid peptide whose amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients.
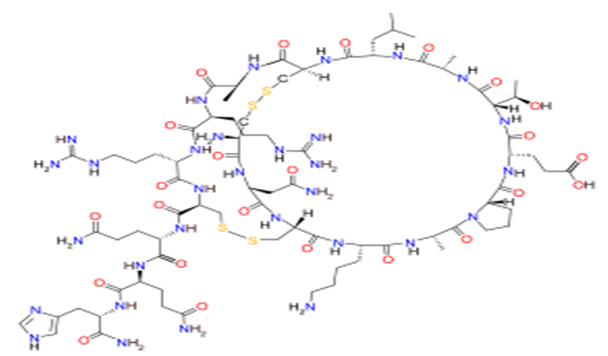
APAMIN - Apaminis an 18 amino acid peptide neurotoxin found in apitoxin (bee venom).7 It selectively blocks SK channels, a type of Ca2+-activated K+ channelexpressed in the central nervous system and smooth muscle. Due to its specificity for SK channels, apamin is used as a drug in biomedical research to study the electrical properties of SK channels and their role in the after hyperpolarizations occurring immediately following an action potential.8
CANNABINOIDS- Cannabinoids are a class of diverse chemical compounds that activate cannabinoid receptors. These include the endocannabinoids (produced naturally in the body by humans and animals),9the phytocannabinoids (found in cannabis and some other plants), and synthetic cannabinoids (produced chemically by humans). The most notable cannabinoid is the phytocannabinoid ?9-tetrahydrocannabinol (THC), the primary psychoactive compound of cannabis.10 However, there are known to exist numerous other cannabinoids with varied effects. Synthetic cannabinoids encompass a variety of distinct chemical classes: the classical cannabinoids structurally related to THC, the nonclassical cannabinoids (cannabimimetics) including the aminoalkylindoles, 1,5-diarylpyrazoles, quinolines, and arylsulphonamides, as well as eicosanoids related to the endocannabinoids.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CHANNELOSOME- The channelosome is the collection of (usually) signalling proteinsassociated with an ion channel. The channelosome is frequently clustered within a lipid microdomain or caveolae. This collection of proteinsmay be involved with anchoring, phosphorylationor some other modulatory or support function. An example is neuralKCNQ/M (Kv7) potassiumchannelosome11
CHOLINE- In general, the word choline refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation. Found in most animal tissues, choline is a primary component of the neurotransmitter acetylcholine and functions with inositolas a basic constituent of lecithin. It prevents fat deposits in the liver and facilitates the movement of fats into the cells. The richest sources of choline are liver, kidney, brain, wheat germ, brewer's yeast, and egg yolk. Therefore, cholinergic typically refers to acetylcholinein the neurological sense. The parasympathetic nervous system, which uses acetylcholine almost exclusively to send its messages, is said to be almost entirely cholinergic. Neuromuscular junctions, preganglionic neurons of the sympathetic nervous system, the basal forebrain, and brain stem complexes are also cholinergic. In addition, the receptor for the merocrine sweat glands are also cholinergic, since acetylcholine is released from post-ganglionic sympathetic neurons.
LIGAND-GATED ION CHANNEL- The Cys-loop ligand-gated ion channelsuperfamily is composed of nicotinic acetylcholine, GABAA, GABAA-ρ, glycine and 5-HT3 receptors that are composed of five protein subunits that form a pentameric arrangement around a central pore. There are usually 2 alpha subunits and 3 other beta, gamma, or delta subunits (some consist of 5 alpha subunits). Cys-loop receptors possess a characteristic loop formed by a disulfide bond between two cysteine (Cys) residues 13 highly conserved amino acids apart near the N-terminal extracellular domain of the alpha subunit. All subunits consist of a conserved extracellular large N-terminal domain, three highly conserved transmembrane domains, a cytoplasmic loop of variable size and amino acid sequence, and a fourth transmembrane domain with a relatively short and variable extracellular C terminal. All alpha subunits have a characteristic cys-cys pair in the N-terminal extracellular domain, this is shown to be essential for agonist binding. The neurotransmitters bind at the interface between subunits in the extracellular domain. Each subunit contains 4-membrane-spanning alpha helixes (M1, M2, M3, M4). The pore is formed primarily by M2 of the two alpha subunits. The M3-M4 linker is the intracellular domain that binds the cytoskeleton12,13
DOPAMINERGIC -Dopaminergicmeans "related to dopamine", a common neurotransmitterin vertebrates.For example, certain proteinssuch as the dopamine transporter(DAT), vesicular monoamine transporter 2(VMAT2), and dopamine receptorscan be classified as dopaminergic, and neuronswhich synthesizeor contain dopamine and synapseswith dopamine receptors in them may also be labeled as dopaminergic. Enzymeswhich regulate the biosynthesisor metabolismof dopamine such as aromatic L-amino acid decarboxylase(AAAD) or DOPA decarboxylase(DDC), monoamine oxidase(MAO), and catechol O-methyl transferase (COMT) may be referred to as dopaminergic as well. Lastly, any endogenousor exogenouschemical substancewhich acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons that synapse onto neurons that release dopamine or express dopamine receptors) can also be said to have dopaminergic effects, two prominent examples being opioidswhich enhance dopamine release indirectly in the reward pathways, and some substituted amphetamines, which enhance dopamine release directly by binding to, and inhibiting VMAT2.
HCN - Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are intermembrane proteins that serve as nonselective ligand-gated cation channels in the plasma membranes of heart and brain cells.14 HCN channels are sometimes referred to as “pacemaker channels” because they help to generate rhythmic activity within groups of heart and brain cells. HCN channels are encoded by four genes (HCN1, 2, 3, 4) and are widely expressed throughout the heart and the central nervous system.15,16 The current flowing through HCN channels, designated If or Ih, plays a key role in the control of cardiac and neuronal rhythmicity ("pacemaker current"). Expression of single isoforms in heterologous systems such as human embryonic kidney (HEK) cells, Chinese hamster ovary (CHO) cells, and Xenopus oocytes yields homotetrameric channels able to generate ion currents with properties similar to those of the native If/Ih current, but with quantitative differences in the voltage-dependence, activation/deactivation kinetics and sensitivity to the nucleotide cyclic AMP (cAMP): HCN1 channels show the more positive threshold for activation, the fastest activation kinetics, and the lowest sensitivity to cAMP, while HCN4 channels are slowly gating and strongly sensitive to cAMP. HCN2 and HCN3 have intermediate properties.17,18,19
HOCPCA- HOCPCA (3-hydroxycyclopent-1-enecarboxylic acid) is a compound with an affinity for the GHB receptor 39 times greater than that of GHB itself.20
HOMOVANILLIC ACID - Homovanillic acid (HOC6H3(OCH3)CH2COOH; synonyms: 3-Methoxy-4-hydroxyphenyl acetic acid; HVA; 4-Hydroxy-3-methoxy-benzeneacetic acid; 4-Hydroxy-3-methoxyphenylacetic acid) is a major catecholamine metabolite. It is used as a reagent to detect oxidative enzymes, and is associated with dopamine levels in the brain. In psychiatry and neuroscience, brain and cerebrospinal fluid levels of HVA are measured as a marker of metabolic stress caused by 2-deoxy-D-glucose.21 HVA presence supports a diagnosis of neuroblastoma and malignant pheochromocytoma. Fasting plasma levels of HVA are known to be higher in females than in males. This does not seem to be influenced by adult hormonal changes, as the pattern is retained in the elderly and post-menopausal as well as transsexuals according to their genetic sex, both before and during cross-sex hormone administration. Differences in HVA have also been correlated to tobacco usage, with smokers showing significantly lower amounts of plasma HVA.22
ION CHANNELS - Ion channels are pore-forming membrane proteins whose functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are considered to be one of the two traditional classes of ionophoric proteins, with the other class known as ion transporters (including the sodium-potassium pump, sodium-calcium exchanger, and sodium-glucose transport proteins, amongst others).23
Ion channels are often studied in the disciplines of biophysics and electrophysiology, utilizing techniques including voltage clamp, patch clamp, immunohistochemistry, and RT-PCR.
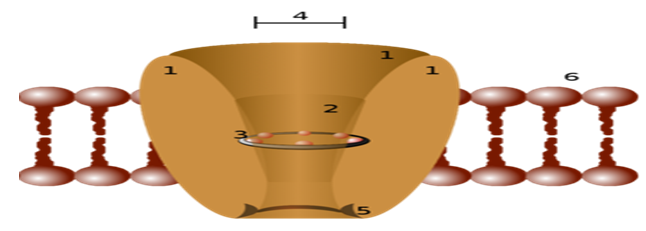
Schematic diagram of an ion channel. 1 - channel domains (typically four per channel), 2 - outer vestibule, 3 - selectivity filter, 4 - diameter of selectivity filter, 5 - phosphorylation site, 6 - cell membrane.
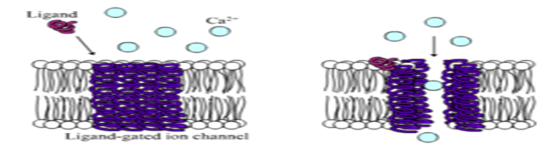
LIGAND-GATED ION CHANNELS(LGICS)- Ligand-gated ion channels (LGICs) are a group of transmembrane ion channel proteins which open to allow ions such as Na+, K+, Ca2+, or Cl- to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand),24 such as a neurotransmitter.25 These proteins are typically composed of at least two different domains: a transmembrane domain which includes the ion pore, and an extracellular domain which includes the ligand binding location (an allosteric binding site). This modularity has enabled a 'divide and conquer' approach to finding the structure of the proteins (crystallising each domain separately). The function of such receptors located at synapses is to convert the chemical signal of presynaptically released neurotransmitter directly and very quickly into a postsynaptic electrical signal. Many LGICs are additionally modulated by allosteric ligands, by channel blockers, ions, or the membrane potential. LGICs are classified into three superfamilies which lack evolutionary relationship: Cys-loop receptors, Ionotropic glutamate receptors and ATP-gated channels. LGICs can be contrasted with metabotropic receptors (which use second messengers), voltage-gated ion channels (which open and close depending on membrane potential), and stretch-activated ion channels (which open and close depending on mechanical deformation of the cell membrane).26,27
LOW-AFFINITY NERVE GROWTH FACTOR RECEPTOR- The Low-Affinity Nerve Growth Factor Receptor (also called the LNGFR or p75 neurotrophin receptor) is one of the two receptor types for the neurotrophins, a family of protein growth factors that stimulate neuronal cells to survive and differentiate. The precise function of the LNGFR is somewhat controversial, in contrast to the function of the high-affinity receptor family for the neurotrophins, the Trk receptor tyrosine kinases such as TrkA.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
NMDA RECEPTOR - The induction of NMDA receptor-dependent long-term potentiation (LTP) in chemical synapses in the brain occurs via a fairly straightforward mechanism28. A substantial and rapid rise in calcium ion concentration inside the postsynaptic cell (or more specifically, within the dendritic spine) is most possibly all that is required to induce LTP. But the mechanism of calcium delivery to the postsynaptic cell in inducing LTP is more complicated. Muscarinic receptors, or mAChRs, are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certain neurons[1] and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system. Muscarinic receptors were named as such because they are more sensitive to muscarine than to nicotine.29 Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpine and scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists.30
NEUROCHEMICAL- A neurochemical is an organic molecule, such as serotonin, dopamine, or nerve growth factor, that participates in neural activity. The science of neurochemistry studies the functions of neurochemicals.
Prominent neurochemicals:The neuropeptide oxytocin. Oxytocin is involved in the control of maternal behavior. It has also been scientifically proven to create feelings of bonding, quench stress, and even speed up wound healing. It is synthesized inside magnocellular neurosecretory cells as a precursor protein that is processed by proteolysis to its shorter active peptide form. Specific parts of the brainsuch as the supraoptic nucleusproduce oxytocin which acts on cells in locations such as the ventral pallidum to produce the behavioral effects of oxytocin. A large amount of oxytocin is made in the hypothalamus, transported to the posterior lobe of the pituitary and released into the blood stream by which it reaches target tissues such as the mammary glands(milk letdown). In the diagram inset, oxytocin is shown bound to a carrier protein, neurophysin.
Glutamate is the most common neurotransmitter. Most neurons secrete with glutamate or GABA. Glutamate is excitatory, meaning that the release of glutamate by one cell usually causes adjacent cells to fire an action potential. (Note: Glutamate is chemically identical to the MSG commonly used to flavor food.) GABA is an example of an inhibitory neurotransmitter. Dopamine is another example of a neurotransmitter. It plays a key role in the functioning of the limbic system, which is involved in emotional function and control. It also plays a part in movement, alertness, and sensations of pleasure. Serotonin plays a regulatory role in mood, sleep, and other areas. Acetylcholine assists motor function and is involved in memory. Nitric oxide functions as a neurotransmitter, despite being a gas. It is not grouped with the other neurotransmitters because it is not released in the same way. Endocannabinoids act in the endocannabinoid system to control neurotransmitter release in a host of neuronal tissues, including the hippocampus, amygdala, basal ganglia, and cerebellum. Eicosanoids act as neurotransmitters via the Arachidonic acid cascade.31
NEUROMODULATION - Neuromodulation is the physiological process by which a given neuron uses several different neurotransmitters to regulate diverse populations of central nervous system neurons. This is in contrast to classical synaptic transmission, in which one presynaptic neuron directly influences a single postsynaptic partner. Neuromodulators secreted by a small group of neurons diffuse through large areas of the nervous system, affecting multiple neurons. Examples of neuromodulators include dopamine, serotonin, acetylcholine, histamine and others. Neuromodulation can be conceptualized as a neurotransmitter that is not reabsorbed by the pre-synaptic neuron or broken down into a metabolite. Such neuromodulators end up spending a significant amount of time in the cerebrospinal fluid (CSF), influencing (or "modulating") the activity of several other neurons in the brain. For this reason, some neurotransmitters are also considered to be neuromodulators, such as serotonin and acetylcholine. Neuromodulation is often contrasted with classical fast synaptic transmission. In both cases the transmitter acts on local postsynaptic receptors, but in neuromodulation, the receptors are typically G-protein coupled receptors while in classical chemical neurotransmission, they are ligand-gated ion channels. Neurotransmission that involves metabotropic receptors (like G-protein linked receptors) often also involves voltage-gated ion channels, and is relatively slow. Conversely, neurotransmssion that involves exclusively ligand-gated ion channels is much faster. A related distinction is also sometimes drawn between modulator and driver synaptic inputs to a neuron, but here the emphasis is on modulating ongoing neuronal spiking versus causing that spiking.
NEUROPROTEOMICS - Neuroproteomics is the study of the protein complexes and species that make up the nervous system. These proteins interact to make the neurons connect in such a way to create the intricacies that nervous system is known for. Neuroproteomics is a complex field that has a long way to go in terms of profiling the entire neuronal proteome. It is a relatively recent field that has many applications in therapy and science. So far, only small subsets of the neuronal proteome have been mapped, and then only when applied to the proteins involved in the synapse.
Neurotrophic factors - Neurotrophic factors are a family of proteins that are responsible for the growth and survival of developing neurons and the maintenance of mature neurons. Recent research has shown that neurotrophic factors promote the initial growth and development of neurons in the central nervous system and peripheral nervous system and that they are capable of regrowing damaged neurons in test tubes and animal models32. Neurotrophic factors are often released by the target tissue in order to guide the growth of developing axons. Most neurotrophic factors belong to one of three families: (1) neurotrophins, (2) glial cell-line derived neurotrophic factor family ligands (GFLs), and (3) neuropoietic cytokines. Each family has its own distinct signaling family though the cellular responses elicited often do overlap. Currently, neurotrophic factors are being intensely studied for use in bioartificial nerve conduits because they are necessary in vivo for directing axon growth and regeneration. In studies, neurotrophic factors are normally used in conjunction with other techniques such as biological and physical cues created by the addition of cells and specific topographies. The neurotrophic factors may or may not be immobilized to the scaffold structure, though immobilization is preferred because it allows for the creation of permanent, controllable gradients. In some cases, such as neural drug delivery systems, they are loosely immobilized such that they can be selectively released at specified times and in specified amounts.
NEUROTROPHINS -Neurotrophins are a family of proteins that induce the survival, development, and function33 of neurons. They belong to a class of growth factors, secreted proteins that are capable of signaling particular cells to survive, differentiate, or grow. Growth factors such as neurotrophins that promote the survival of neurons are known as neurotrophic factors. Neurotrophic factors are secreted by target tissue and act by preventing the associated neuron from initiating programmed cell death - thus allowing the neurons to survive. Neurotrophins also induce differentiation of progenitor cells, to form neurons. Although the vast majority of neurons in the mammalian brain are formed prenatally, parts of the adult brain (for example, the hippocampus) retain the ability to grow new neurons from neural stem cells,a process known as neurogenesis. Neurotrophins are chemicals that help to stimulate and control neurogenesis.34
SEROTONERGIC- Serotonergicor serotoninergic means "related to the neurotransmitter serotonin". A synapse is serotonergic if it uses serotonin as its neurotransmitter. A substance is serotonergic if it produces its effects via interactions with the serotonin system. A serotonergic, or serotonergic agent, is any chemical which functions to enhance the effects mediated by serotonin in the central nervous system, and they include the following classes of chemicals:
- Serotonin precursors (such as tryptophanand 5-HTP)
- Cofactors required in the body's production of serotonin
- Serotonergic enzymes
- Serotonin antagonist and reuptake inhibitor
- Serotonin reuptake inhibitor- A class of serotonergic antidepressants
- Noradrenergic and specific serotonergic antidepressant- Another class of serotonergic antidepressants
- Serotonergic psychedelics- The serotonergic psychedelic drugs
SIGNAL TRANSDUCTION- Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response.35 There are two stages in this process:
- A signaling molecule activates a specific receptor protein on the cell membrane.
- A second messenger transmits the signal into the cell, eliciting a physiological response.
In either step, the signal can be amplified. Thus, one signalling molecule can cause many responses.36 A signal transduction functions much like a switch.
SK CHANNELS - SK channels (Small conductance calcium-activated potassium channels) are a subfamily of Ca2+-activated K+ channels.37They are so called because of their small single channel conductance in the order of 10 pS. SK channels are a type of ion channel allowing potassium cations to cross the cell membrane and are activated (opened) by an increase in the concentration of intracellular calcium through N-type calcium channels. Their activation limits the firing frequency of action potentials and is important for regulating afterhyperpolarization in the neurons of the central nervous system as well as many other types of electrically excitable cells. This is accomplished through the hyperpolarizing leak of positively charged potassium ions along their concentration gradient into the extracellular space. This hyperpolarization causes the membrane potential to become more negative.38 SK channels are thought to be involved in synaptic plasticity and therefore play important roles in learning and memory.39
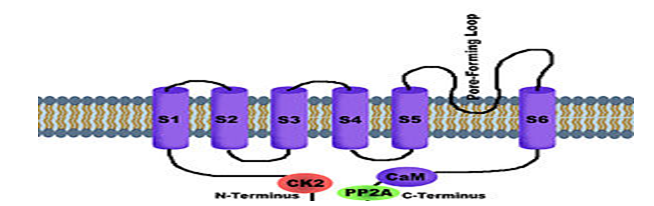
SK3 - SK3 (small conductance calcium-activated potassium channel 3) also known as KCa2.3 is a protein that in humans is encoded by the KCNN3 gene.40 SK3 is a small-conductance calcium-activated potassium channel partly responsible for the calcium-dependent after hyperpolarisation current (IAHP). It belongs to a family of channels known as small-conductance potassium channels, which consists of three members – SK1, SK2 and SK3 (encoded by the KCNN1, 2 and 3 genes respectively), which share a 60-70% sequence identity. These channels have acquired a number of alternative names, however a NC-IUPHAR has recently achieved consensus on the best names, KCa2.1 (SK1), KCa2.2 (SK2) and KCa2.3 (SK3).Small conductance channels are responsible for the medium and possibly the slow components of the IAHP.
SoRI-20041 - SoRI-20041 is an "antagonist-like" allosteric modulator of amphetamine-induced dopamine release[1] (in contrast to the related research chemicals SoRI-9804 and SoRI-20040, which are "agonist-like"), SoRI-20041 is believed to be the first example of a drug that separately modulates uptake versus release in the dopamine transporter (possibly showing how inward and outward transport represent distinct operational modes of DAT); it produced the same effects as SoRI-20040 and SoRI-9804 in uptake assays and binding assays, inhibiting the re-uptake of dopamine, but did not modulate d-amphetamine-induced DA release by inhibiting that as well, like 'agonists' of the series do.41 This suggests the possibility of simultaneous action & increase of indirect-agonism through the dual-action of DRA & DRI efficacy existing together. Greatening of the inhibition of re-uptake at synaptic dopamine concentrations without interference to the flow of release of dopamine from amphetaminergic phosphorylation at the transporter so effected. This overcomes the obstacle of a compromised binding site which would be rendered unusable through the action of amphetamine. A site which conventional dopamine re-uptake inhibitors (such as cocaine or methylphenidate) would otherwise ineffectively target on each specific transporter so effected by amphetamine. Making this an example of a DRI that does not have a mutually exclusive functionality against DRA action at individual instances of DAT.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TRACE AMINES -Trace amines are an endogenous group of amines structurally and metabolically related to classical monoamine neurotransmitters, such as dopamine, norepinephrine, and serotonin. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although, they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems.42 Trace amines may play very significant roles in the coordination of biogenic monoamine-based synaptic physiology. At high concentrations, they have well-characterized presynaptic ‘‘amphetamine-like’’ effects on monoamine release, reuptake and biosynthesis; at lower concentrations, they possess postsynaptic modulatory effects that potentiate the activity of other neurotransmitters, particularly dopamine and serotonin.43A family of G protein coupled receptors known as TAARs (trace amine associated receptors) has been characterized to be responsive to trace amines44 and structurally related psychoactive drugs, such as amphetamine, MDMA, LSD, and DMT.45 Like dopamine, noradrenaline, and serotonin, the trace amines have been implicated in a vast array of human disorders of affect and cognition, such as depression46 and schizophrenia.47 A thorough review of trace amines and trace amine receptors that discusses the historical evolution of this research particularly well is that of Grandy.48
ULTRADIANRHYTHMS - Ultradian rhythms are recurrent periods or cycles repeated throughout a 24-hour circadian day. In contrast, infradian rhythms, such as the human menstrual cycle, have periods longer than a day. The descriptive term ultradian is used in sleep research in reference to the 90–120 minute cycling of the sleep stages during human sleep.49 Some of the other ultradian cyclings of the body are blood circulation, blinking, pulse, hormonal release, heart rate, thermoregulation, urination, bowel activity, nostril dilation and appetite. The last involves rhythmic release of Neuropeptide Y (NPY) and Corticotropin-releasing hormone (CRH), stimulating and inhibiting appetite ultradian rhythms. Caenorhabditis elegans is often used as a model animal for ultradian rhythm. Defecation in C. elegans is a tightly controlled rhythmic process. Posterior body wall muscle contractions in C. elegans occur rhythmically every 45–50 seconds and mediate defecation. Ultradian mood states in bipolar disorder cycle much faster than rapid cycling; the latter is defined as four or more mood episodes in one year, sometimes occurring within a few weeks. Ultradian mood cycling is characterized by cycles shorter than 24 hours.50
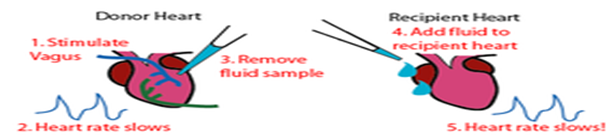
VAGUSSTOFF- Vagusstoff (literally translated from German as "Vagus Substance") refers to the substance released by stimulation of the vagus nervewhich causes a reduction in the heartrate. Discovered in 1921 by physiologist Otto Loewi, vagusstoff was the first confirmation of chemical synaptic transmissionand the first neurotransmitterever discovered. It was later confirmed to be acetylcholine, which was first identified by Sir Henry Hallett Dalein 1914. Because of his pioneering experiments, in 1936 Loewi was awarded the Nobel Prize in Physiology or Medicine, which he shared with Dale.
Loewi's experiment proving that neurotransmision was chemical, rather than electrical.
VANILLYL MANDELIC ACID(VMA) -Vanillyl mandelic acid (VMA) is an end-stage metabolite of the catecholamines epinephrine and norepinephrine. It is produced via intermediary metabolites. VMA is found in the urine, along with other catecholamine metabolites, including homovanillic acid (HVA), metanephrine, and normetanephrine. In timed urine tests the quantity excreted (usually per 24 hours) is assessed along with creatinine clearance, and the quantity of cortisols, catecholamines, and metanephrines excreted is also measured.
VESICULAR MONOAMINE TRANSPORTER(VMAT)-
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membrane of intracellular vesicles of presynaptic neurons. It acts to transport monoamines into the synaptic vesicles.
CONCLUSION
The study of the chemical composition and processes of the nervous system and the effects of chemicals on it is the basic concept of neurochemistry Multiple neurochemical processes are involved in the synthesis, packaging, and release of neurotransmitters, and in the production and function of neurotransmitter receptors. Significantly, each of these biochemical steps represents a point of potential regulation of synaptic function and a site of possible age-related changes. Most neurons produce and release one of several small molecules that serve as neurotransmitters, including acetylcholine, biogenic amines (dopamine, norepinephrine, epinephrine, histamine, or serotonin), or amino acids (glutamate, glycine, or gamma-aminobutyric acid). Many neurons also release one or more neuroactive peptides (neuropeptides), which provide additional modulation of signal transmission. Low levels of neuronal activity often result in release of only the small-molecule transmitter, whereas higher levels of activity result in the co-release of neuropeptides. Release of the neuropeptides may cease at very high levels of activity, however, since peptides must be delivered from the cell body and are replenished slowly. In contrast, synthesis and packaging of other neurotransmitters occurs more rapidly because the necessary synthetic enzymes are present within the cytoplasm in the region of the synapse. The release of neurotransmitters depends upon an increase in intracellular calcium that occurs with the depolarization (decrease in membrane potential) associated with the arrival of action potentials, regenerative waves of electrical activity that are the basis for signaling along neronal processes. Increased calcium leads to modification of vesicle-binding proteins, which facilitate the fusion of vesicles, membrane-bound packages in the cytoplasm, with the cell membrane and subsequent release of the vesicles' contents into the extracellular space.
REFERENCES
1.Siegel, George J.; Albers, R.W., Brady, S.T., Price, D.L. (2006). Basic Neurochemistry, 7th Ed.
2.Foye, William O. (2007). Foye's Principles of Medicinal Chemistry. David A. Williams. Lippincott Williams & Wilkins. p. 446.
3. D'haenen, Hugo (2002) (digitised online by Google books). Biological Psychiatry. Paul Willner. John Wiley and Sons. p. 415.
4 .Hynes, T. R.; Randal, M.; Kennedy, L. A.; Eigenbrot, C.; Kossiakoff, A. A. (1990). "X-ray crystal structure of the protease inhibitor domain of Alzheimer's amyloid beta-protein precursor". Biochemistry 29 (43): 10018–10022.
5. Wang Y, Ha Y (August 2004). "The X-ray structure of an antiparallel dimer of the human amyloid precursor protein E2 domain". Mol. Cell 15 (3): 343–53.
6. Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (July 2006). "Synapse formation and function is modulated by the amyloid precursor protein". J. Neurosci. 26 (27): 7212–21
7. Habermann E (1984). "Apamin". Pharmacol. Ther. 25 (2): 255–70.
8. Castle NA, Haylett DG, Jenkinson DH (February 1989). "Toxins in the characterization of potassium channels". Trends Neurosci. 12 (2): 59–65.
9.Pacher P, Batkai S, Kunos G (2006). "The Endocannabinoid System as an Emerging Target of Pharmacotherapy". Pharmacol Rev. 58 (3): 389–462.
10. Lambert DM, Fowler CJ (2005). "The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications". J. Med. Chem. 48 (16): 5059–87.
11. 11. Delmas, P.; Brown, D. A. (2005). "Pathways modulating neural KCNQ/M (Kv7) potassium channels". Nature Reviews Neuroscience 6 (11): 850.
12.Sine S, Engel A (2006). "Recent advances in Cys-loop receptor structure and function.". Nature 440 (7083): 448–55. doi:10.1038/nature04708. PMID 16554804.
13.Albuquerque et al. (2009). "Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function". Physiol Rev 89 (1): 73–120.
14.Luthi A, McCormick DA. 1998. Neuron. H-current: properties of a neuronal and network pacemaker. Vol. 21. pp 9-12.
15. Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels (2001)Annu Rev Physiol. 63:235-57. Review.
16. Notomi T, Shigemoto R (2004) Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471: 241-276.
17. Wahl-Schott C, Biel M. (2009) HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci. 2009 Feb;66(3):470-94. Review
18. Baruscotti, M., Bucchi, A., DiFrancesco, D. (2005). Physiology and pharmacology of the cardiac pacemaker ("funny") current. Pharmacology & Therapeutics, 107, 59-79
19. Santoro B, Tibbs GR. (1999) The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci. Apr 30;868:741-64. Review
20. Petrine Wellendorph, Signe Høg, Jeremy R. Greenwood, Anne de Lichtenberg, Birgitte Nielsen, Bente Frølund, Lotte Brehm, Rasmus P. Clausen and Hans Bräuner-Osborne (2005). "Novel Cyclic γ-Hydroxybutyrate (GHB) Analogs with High Affinity and Stereoselectivity of Binding to GHB Sites in Rat Brain" (pdf). The Journal of Pharmacology and Experimental Therapeutics 315 (1): 346–351
21. Marcelis M, Suckling J, Hofman P, Woodruff P, Bullmore E, van Os J (September 2006). "Evidence that brain tissue volumes are associated with HVA reactivity to metabolic stress in schizophrenia". Schizophr. Res. 86 (1–3): 45–53.
22. Giltay E, Kho K, Blandjaar B, Verbeek M, Geurtz P, Geleijnse J, Gooren L (July 2005). "The sex difference of plasma homovanillic acid is unaffected by cross-sex hormone administration in transsexual subjects". J Endocrinol 187 (1): 109–16.
23. Hille, Bertil (2001) [1984]. Ion Channels of Excitable Membranes (3rd ed.). Sunderland, Mass: Sinauer Associates, Inc.. pp. 5
24. Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed.. Sinauer Associates. pp. 156–7.
25. Connolly CN, Wafford KA (2004). "The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function". Biochem. Soc. Trans. 32 (Pt3): 529–34.
26.Cascio M (2004). "Structure and function of the glycine receptor and related nicotinicoid receptors". J. Biol. Chem. 279 (19): 19383–6.
27. Collingridge GL, Olsen RW, Peters J, Spedding M (January 2009). "A nomenclature for ligand-gated ion channels". Neuropharmacology 56 (1): 2–5.
28.Eglen RM (July 2006). "Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function". Auton Autacoid Pharmacol 26 (3): 219–33.
29. Ishii M, Kurachi Y (2006). "Muscarinic acetylcholine receptors". Curr. Pharm. Des. 12 (28): 3573–81.
30.Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed.. Sinauer Associates. pp. 122–6
31. Piomelli, Daniele (2000). "Arachidonic Acid". Neuropsychopharmacology: The Fifth Generation of Progress.
32. Deister, C. and C.E. Schmidt, Optimizing neurotrophic factor combinations for neurite outgrowth. Journal of Neural Engineering, 2006. 3: p. 172-179.
33. Hempstead BL (February 2006). "Dissecting the diverse actions of pro- and mature neurotrophins". Curr Alzheimer Res 3 (1): 19–24.
34. Reichardt LF (September 2006). "Neurotrophin-regulated signalling pathways". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361 (1473): 1545–64.
35. Silverthorn (2007). Human Physiology. 4th ed.
36.Reece, Jane; Campbell, Neil (2002). Biology. San Francisco: Benjamin Cummings.
37. Bond CT, Maylie J, Adelman JP (1999). "Small-conductance calcium-activated potassium channels". Ann. N. Y. Acad. Sci. 868: 370–8.
38. Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP (1996). "Small-conductance, calcium-activated potassium channels from mammalian brain". Science 273 (5282): 1709–14.
39. Faber ES, Sah P (2007). "Functions of SK channels in central neurons". Clin. Exp. Pharmacol. Physiol. 34 (10): 1077–83
40. Chandy KG, Fantino E, Wittekindt O, Kalman K, Tong LL, Ho TH, Gutman GA, Crocq MA, Ganguli R, Nimgaonkar V, Morris-Rosendahl DJ, Gargus JJ (January 1998). "Isolation of a novel potassium channel gene hSKCa3 containing a polymorphic CAG repeat: a candidate for schizophrenia and bipolar disorder?". Mol. Psychiatry 3 (1): 32–7.
41. Rothman, R. B.; Dersch, C. M.; Ananthan, S.; Partilla, J. S. (2009). "Studies of the Biogenic Amine Transporters. 13. Identification of “Agonist” and “Antagonis” Allosteric Modulators of Amphetamine-Induced Dopamine Release" (pdf). The Journal of Pharmacology and Experimental Therapeutics 329 (2): 718–728
42. Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006 Aug;79(5-6):223-46.
43.Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006 Aug;79(5-6):223-46.
44.Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005 Mar;85(3):372-85.
45.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001 Dec;60(6):1181-8.
46.Davis, B.A., Boulton, A.A., 1994. The trace amines and their acidic metabolites in depression—an overview. Prog. Neuropsychopharmacol. Biol. Psychiatry 18, 17–45.
47.O’Reilly, R.L., Davis, B.A., 1994. Phenylethylamine and schizophrenia. Prog Neuropsychopharmacol. Biol. Psychiatry 18, 63–75.
48.D. K. Grandy (2007). "Trace amine-associated receptor 1—Family archetype or iconoclast?" Pharmacology & Therapeutics 116 (3) 355-390.
49. Hobson JA, Pace-Schott EF (September 2002). "The cognitive neuroscience of sleep: neuronal systems, consciousness and learning". Nat. Rev. Neurosci. 3 (9): 679–93.
50. Kramlinger KG, Post RM (March 1996). "Ultra-rapid and ultradian cycling in bipolar affective illness". Br J Psychiatry 168 (3): 314–23.
51. Magera MJ, Thompson AL, Matern D, Rinaldo P (May 2003). "Liquid chromatography-tandem mass spectrometry method for the determination of vanillylmandelic acid in urine". Clin. Chem. 49 (5): 825–6
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









