About Author
Nrusingha Panda**
Department of Quality Assurance, Mankind Pharma Ltd., Bermiok Elaka, South Sikkim, Sikkim, Pin code- 737126, India
ABSTRACT
Cleaning validation is a regulatory requirement for pharmaceutical industries. For performing cleaning validation, Pharma industries follow different regulatory guidelines out of which Active Pharmaceutical Ingredients Committee (APIC) is one which has shown what approach should be followed for worst case finding in a matrix and how cleaning validation should be performed, specifically in Active Pharmaceutical Ingredient (API) manufacturing industries. This article focuses on some issues related to the worst case rating procedure mentioned in the APIC guideline.
INTRODUCTION
Many pharmaceutical companies especially those pharmaceutical industries associated with manufacturing of active pharmaceutical ingredients (API) mainly follow the guideline presented by Active Pharmaceutical Ingredients Committee (APIC). APIC has published its guideline on cleaning validation in May, 2014 titled “Guidance on aspect of cleaning validation in active pharmaceutical ingredient plants”. It revised its guideline in September, 2016 to include “Health based exposure limit (HBEL)” criteria as mentioned in the European Medicines Agency (EMA) guideline on cleaning validation. APIC has included the Permitted daily exposure (PDE) based calculation of previous product in the next product. EMA is the front runner in adopting the HBEL based calculation in its guideline and since then many drug regulators of different countries have adopted the same approach. APIC guideline is being followed by many pharmaceutical formulation industries also for cleaning validation in their facilities. Current version of APIC guideline has mentioned that the processes taking place in API manufacturing companies are different than that taking place in finished formulation industries and higher limits of carryover may be acceptable in API manufacturing plants because the carry over risk is much lower for technical and chemical manufacturing reasons. So before adopting the approach mentioned in the APIC guideline, finished formulation industries should take in to consideration for different processes that take place at respective stages of manufacturing and cleaning processes. Since cleaning validation is an important aspect of Good manufacturing practices (GMP) at any regulated manufacturing facility of any finished formulation, it is important to know the pros and cons of any guideline before being adopted. APIC has done the same mistake in its current version guideline as it did in its previous version since it has carried the same mistake in worst case product approach and calculation of maximum allowable carry over (MACO). APIC has adopted a more relaxation approach than any other regulatory guideline on MACO calculation.
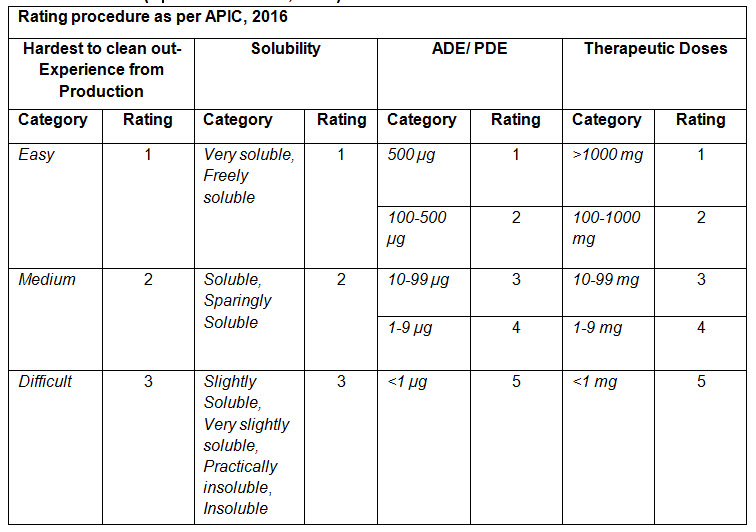
Rating procedure for worst case product mentioned in APIC guideline, 2016: APIC, 2016 has assigned rating for different categories in different criteria such as Hardest to clean out, Solubility, ADE/PDE and Therapeutic doses criteria.
• Hardest to Clean out criteria:
In this criterion, hardest or difficult to clean out products are find out based on the experience and interview from production operator and supervisor. The rating value increases with the increase in difficult to clean out the product.
• Solubility:
In this criterion, rating is given by taking descriptive terms of solubility in United States Pharmacopoeia (USP). The rating increases with the increase in insolubility of the substance in the solvents used for cleaning.
• ADE/PDE:
Here, based on the ADE/ PDE of a substance, a rating is given. The rating increases as the ADE/PDE value decreases.
• Therapeutic Dose:
Here, based on the Therapeutic dose of a substance, a rating is given. The rating increases as the therapeutic dose value decreases.
A summary of all of the above ratings are mentioned in the below table (Table-1) :
Table-1 (Reference: APIC, 2016)
Worst case rating procedure given in the guideline: The substances are scientifically matrixed by equipment class (train/equipment) and cleaning class (procedure). Each existing combination of the classes is considered as a group. When this bracketing has been carried out, the - “Worst Case Rating (WCR)”- can start. For at least one worst case in each group, cleaning validation studies shall be carried out. As an example, train A has been selected mentioned in the point no. 7.2 of the guideline.

A matrix approach is followed for train A where two cleaning classes are identified for cleaning all the substances produced in Train A. Details are given in the below table (Table-2):
Table-2 (Reference: APIC, 2016)
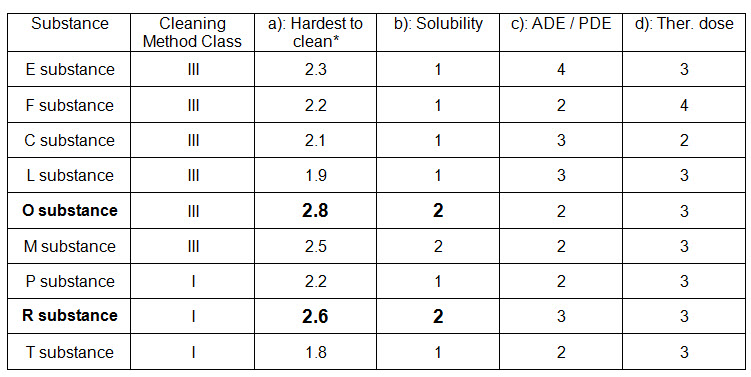
* Each figure is the mean value for different questions answered by operators and supervisors.
For the products in this train two cleaning methods (Class I and III) are used.
Therefore two groups have to be validated. The worst case product (for the validation study) for class III is O substance (Solubility 2 and Hardest to clean* 2.8).
The worst case product (for the validation study) for class I is R substance (Solubility 2 and Hardest to clean* 2.6).
In both cases the limit should be calculated with the most active substance (ADE 4).
If ADE / PDE data are not available, the limit should be calculated with the most active substance (Therapeutic dose 4).
If the limit calculated with ADE / PDE 4 or Therapeutic dose 4 is achievable for all products, this limit can be chosen for both groups.
If the limit calculated with ADE 4 or Therapeutic dose 4 is too low and not achievable for all products, E substance and F substance should be considered as separate groups or produced in dedicated equipment.
The limit for the remaining group should be calculated with the most active substance (ADE / PDE 3 or Therapeutic dose 3).
In case a substance of top priority is not produced regularly, the substance with the second highest priority will be tested in order to show that the cleaning procedure is sufficient for all the other substances in that class. The substance of top priority will then be tested at the first possible occasion.
Formula for Calculation of residue limit of previous product in the next product :
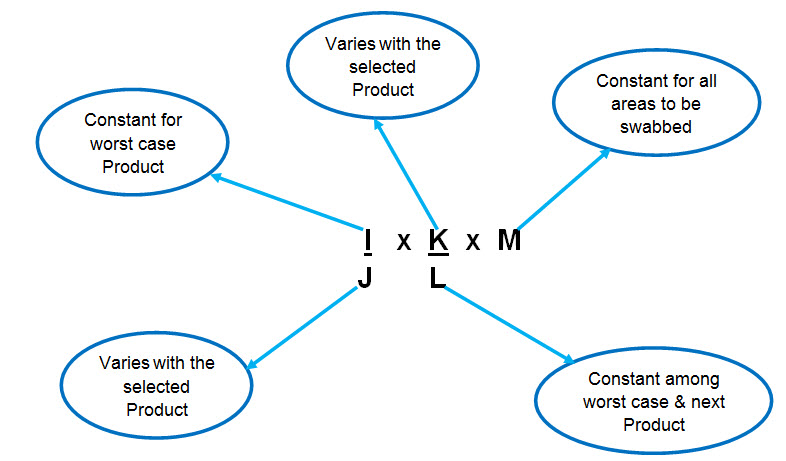
Where, I= 1/1000 of minimum daily dose of previous product or 10 ppm or ADE/ PDE of Previous product
J= Largest daily dose of next product
K= Minimum batch size of next product
L= Shared surface area of equipments
M= Swab area
Relaxation in the Worst case rating: Let us take an example similar to that mentioned in table-2. Suppose in an equipment train, products A, B, C, D, E & F are manufactured and all these products are cleaned by using only one cleaning method i.e. Class I. The details of matrixing are shown in Table-3.
Table-3 (Reference: An example cited following rating procedure as mentioned in APIC, 2016)
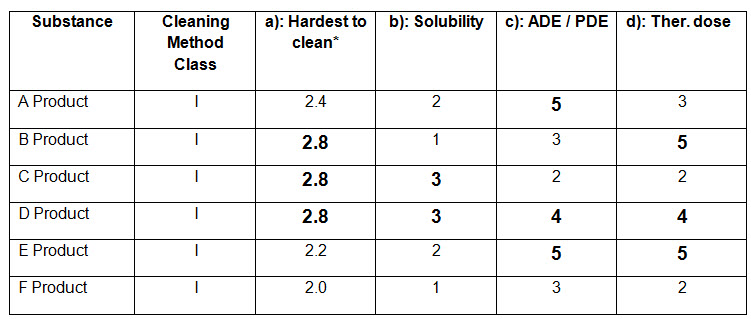
From the above table (Table-3), Hardest to clean products are B, C and D. Solubility ratings of product B, C and D are 1, 3 and 3 respectively. Since product C and D have higher rating i.e. 3, and same rating for “Hardest to clean” (i.e. 2.8), how can one select the worst case among products C and D and on what basis one can select the worst case?
If somehow, the worst case product is selected, then what will be the criteria of selecting the next considered product for calculation of the residue limit of previous product?
Suppose product C has been selected as the worst case product, then among product A and E (most active products since ADE=5), which product shall be considered as the next product?
Suppose ADE/ PDE data is not available, then based on therapeutic dose, product B and E are the most active (since therapeutic dose rating= 5). So, among product B and E, which product will be selected as next considered product?
Let’s see an example by taking product B and E as next considered product individually:
Suppose Product B has a largest daily dose of 100 mg and batch size as 100 kg and product E has a largest daily dose of 200 mg and batch size as 50 kg.
Suppose product C (which is the worst case) is having a minimum daily dose as 10 mg and batch size 50 kg.
Suppose common surface area of equipments for production of products B, C and E are 1000000 cm2 and swab area is 25 cm2.
Residue limit calculation of Worst case product C based on therapeutic dose criteria:

From the above calculations of two residue limits, 0.25 mg is the minimum. So, product B should be selected as the next considered product for calculation purpose.
From the above examples, we can see that there is no relation of the rating of a product based on ADE/ PDE or Therapeutic dose category that it can be selected as the next product for calculation purpose.
Now, consider the same example given in the table-3. Suppose rating of product B based on Therapeutic dose is 4 instead of 5 and that of product E remains same (i.e. 5). So Product E becomes most active (rating = 5). So, in this case, product E should be selected as the next product for calculation purpose. But, the residue limit is calculated as 3.1 mg by taking product E as the next product which is much higher than that calculated by taking product B as the next product (residue limit calculated= 0.25 mg).
That’s a big question to be asked as far as the calculation of residue limit of previous product is concerned.
Conclusion:
APIC guideline, 2016 mentioned a lot of basic things to be known about cleaning validation. In API manufacturing industries, the guidance can be followed having some relaxation in worst case rating procedure since the cleaning procedure in API manufacturing industries is totally different than that in formulation industries. The relaxation in worst case rating procedure cited by APIC may not be adopted by the formulation industries, rather they should follow stringent worst case rating procedure.
REFERENCES :
1. Guidance on aspects of cleaning validation in active pharmaceutical ingredient plants, 2016.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









