About Author:
Tarika Priya
SLC's college of pharmacy. JNTUH
Hyderabad-500076
a4aryatarikapriya@gmail.com
1. Abstract
The aim of study was to screen the antibacterial and antifungal activities of methanol and ethanol extracts of fenugreek seed (Trigonella foenum). Crude extracts of the seeds with methanol and ethanol were screened for antibacterial activities against two gram positive pathogenic bacteria – Bacillus subtilis, Staphylococcus aureus and against two gram negative pathogenic bacteria – E.coli, klebsiella. The antibacterial activity was performed using cup-plate method. The extracts were prepared using soxhlet extraction method. Methanol extract showed activity against E.coli and Klebsiella were as ethanol extract showed activity against Staphylococcus and Bacillus spp.,
The anti fungal activity was also screened against Aspergillus niger. Both the extracts showed good activity against the fungi.
The results obtained in the present study suggest that the extracts revealed a significant scope to develop a novel broad spectrum of antibacterial herbal formulations.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1364
2. INTRODUCTION
Natural products have been a major source of new drugs. Plants are used medicinally in different countries and are a source of many potent and powerful drugs. Medicinal plants are used by 80% of the world population as the only available medicines especially in developing countries. Current research on natural molecules and products primarily focuses on plants since they can be sourced more easily and be selected based on their ethno-medicinal uses.
A wide range of medicinal plants parts is used to extract as raw drugs and they possess varied medicinal properties. While some of these raw drugs are collected in smaller quantities by the local communities and folk healers for local use, many other raw drugs are collected in larger quantities and traded in the market as the raw materials for many herbal industries. Plants used for traditional medicine contain a wide range of substances that can be used to treat chronic as well as infectious diseases. Clinical microbiologists have great interest in screening of medicinal plants for new therapeutics. The active principles of many drugs found in plants are secondary metabolites. The antimicrobial activities of plant extracts may reside in a variety of different components, including aldehyde and phenolic compounds. The development of drug resistance in human pathogens against commonly used antibiotics has necessitated a search for new antimicrobial substances from other sources including plants Screening of medicinal plants for antimicrobial activities is important for finding potential new compounds for therapeutic uses.
Spices and herbs have been used for thousands of centuries by many cultures and scientific experiments have documented the antimicrobial properties of spices. Fenugreek is commonly used spice in Bangladesh.
Fenugreek (Trigonella foenum graecum) is an annual herb that belongs to the family Leguminosae widely grown in Pakistan, India, Egypt, and Middle Eastern countries. Due to its strong flavor and aroma fenugreek in one of such plants whose leaves and seeds are widely consumed in Indo-Pak subcontinent as well as in other oriental countries as a spice in food preparations, and as an ingredient in traditional medicine. It is rich source of calcium, iron, carotene and other vitamins.
[adsense:468x15:2204050025]
Fenugreek seeds have been found to contain protein, vitamin C, niacin, potassium, and diosgenin (which is a compound that has properties similar to estrogen). Other active constituents in fenugreek are alkaloids, lysine and L-tryptophan, as well as steroidal saponins (diosgenin, yamogenin, tigogenin, and neotigogenin).

The fenugreek seeds are rich in dietary fiber, that it can lower blood sugar levels in diabetes.Fenugreek seed is widely used as a galactagogue that is often used to increase milk supply in lactating women and to cure breast cancer. Fenugreek seed is useful for tuberculosis, diabetes, atherosclerosis, constipation, high cholesterol, hypertriglyceridemia and externally it is used as a poultice for abscesses, boils, carbuncles, etc.
The seeds of fenugreek herb possess toxic oils, and other bioactive constituents of the fenugreek seed include volatile oils and alkaloids in it have been shown to be toxic to bacteria parasites and fungi. Recent pharmacological investigation of the seed extract of this plant showed anticancer properties.
Based on the studies carried out in fenugreek, worldwide report shows that the seeds of this plant possess strong antibacterial activity. The potential for developing antibacterial into medicine appears rewarding from both the perspective of drug development and the perspective of phytomedicine.
3. RESEARCH ENVISAGED
Fenugreek contains steroidal saponins, occurring mainly as furostanol 3,26-diglycosides such as trigofoenosides A-G [2-10]. On hydrolysis the saponins yield 0.6-1.7% of spirostanol, sapogenins consisting mainly (about 95%) of diosgenin and its 25β-epimer yamogenin in a 3:2 ratio, together with tigogenin and others. Steroidal saponin peptide esters such as fenugreekine are also present.
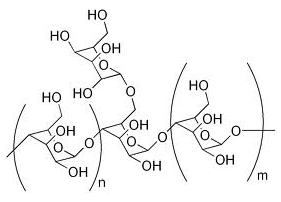
Mucilage polysaccharide, consisting mainly of galactomannan (25-45%) with a backbone of β-(1→4) linked manose residues, branches of α-(1→6) galactosyl residues and a small portions of xylose.
Other constituents include: trigonelline (=coffearine, the N-methyl betaine of nicotinic acid) protein (rich in tryptophan and lysine), free amino acids, principally 4-hydroxyisoleucine, saponin hydrolyzing enzymes, proteinase inhibitors which act on human trypsin and chymotrypsin, scopoletin and other coumarins, flavone glycosides, sterols (cholesterol, β-sitosterol), lecithin and choline.
A small amount (<0.01%) of volatile oilis present, in which alkanes, terpenes, oxygenated and aromatic compounds have been identified. The dominant and characteristic aroma compound in fenugreek is 3-hydroxy-4-5-dimethyl-2(5H)-furanone (sotalone) of which 3-25mg/kg is present in the seeds.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
STEROIDAL SAPONINS OF FENUGREEK
The presence of special steroidal substances in Fenugreek seeds was first reported in 1919 by Wunschendorff, a French research scientist working in Algeria. This discovery was confirmed in various follow-up studies notably by Marker et al, who detected the presence of diosgenin, gitogenin and traces of tigogenin after hydrolysis of the plant material.
Fenugreek seeds contain saponins in the form of furostanol saponins. Furostanol saponins may be defined as bidesmosidic saponins which have two sugar chains, with one bonded at C3 and one attached through an ether linkage at C26 with a D-glucose3.
Fenusterol is the saponin rich fraction obtained from Fenugreek seeds. Saponins are complex glycosidic compounds present in a diverse array of edible and inedible plants1. Each saponin consists of a sapogenin which constitutes the aglycon moiety of the molecule, and a sugar. The sapogenin may be a steroid or a triterpene and the sugar moiety may be glucose, galactose, pentose or a methylpentose.
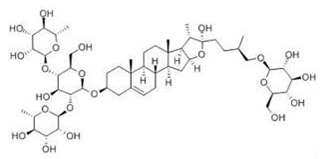
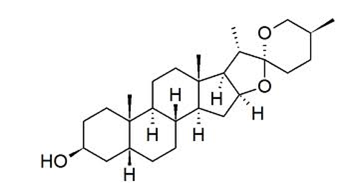
Fenugreek seeds have been found to contain at least a dozen different other saponins. The main saponin is diosgenin and its isomers yamogenin, gitogenin and tigogenin. The other furostanols include smilagenin, sarsasapogenin, neotigenin, yuccagenin, lilagenin and neogitogenin.
Diosgenin, a steroidsapogeninin fenugreek, is the product of hydrolysis by acids, strong bases, or enzymes of saponins. Diosgenin is the precursor for the semi synthesisof progesterone which in turn was used in early combined oral contraceptive pills.The unmodified steroid has estrogenicactivityand can reduce the level of serumcholesterol.
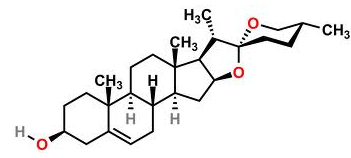
Yamogenin is a chemical compoundof the class calledsapogenins.
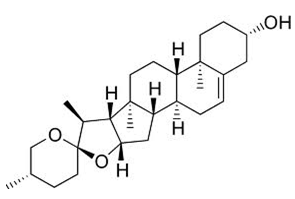
Sarsasapogenin is a steroidalsapogenin, that is the aglycosidic portion of a plant saponin. Sarsasapogenin and its C-25 epimer smilageninlowered blood sugar and reversed diabetic weight gain in experiments within mice with a mutant diabetesgene (db). Both steroids also halted the decline in muscarinic acetylcholine receptors(mAChRs) in animal models of Alzheimer's disease.
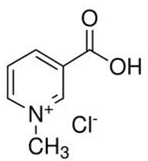
Trigonelline is an alkaloidwith chemical formulaC7H7NO2. It is a zwitterionformed by the methylationof the nitrogen atom of niacin(vitamin B3). Trigonelline is a product of niacin metabolism that is excreted in urine.A major ingredient of coffee, trigonelline is believed to have anti-migraine, anti-carcinogenic, antiseptic, hypoglycemic and hypocholesterolemic activities. It may also act as a stimulant and sedative.Trigonelline has both anti-bacterial and anti-adhesive properties that can help prevent dental cavities from forming.
Galactomannans are polysaccharidesconsisting of a mannosebackbone with galactoseside groups (more specifically, a (1-4)-linked beta-D-mannopyranose backbone with branchpoints from their 6-positions linked to alpha-D-galactose, i.e. 1-6-linked alpha-D-galactopyranose).Galactomannans are used in foods as stabilisers.
Studies show that the saponin rich dry ethanolic extracts of fenugreek slightly inhibited the growth of fungi and bacteria. It was also found that the dry ethanolic extract of fenugreek showed antimicrobial activity at higher doses.
However, to the best of our knowledge very few reports are available on antibacterial properties of fenugreek seed against human pathogenic bacteria so far. Therefore, in the present investigation attempts were made to isolate and investigate the antibacterial activities of Fenugreek extracts.
4. RESUME AND DISCUSSION
The extraction and anti microbial studies were carried out and discussed under the following heads.
A. EXTRACTION OF FENUGREEK SEEDS.
B. ANTIMICROBIAL STUDIES
A .EXTRACTION OF FENUGREEKSEEDS
Two extactions of fenugreek were prepared using methanol and ethanol as solvents. The Extraction was done using the soxhlet extractor.
A Soxhlet extractor is a piece of laboratoryapparatus [1]invented in 1879 by Franz von Soxhlet. It was originally designed for the extraction of a lipidfrom a solid material. However, a Soxhlet extractor is not limited to the extraction of lipids. Typically, a Soxhlet extraction is only required where the desired compound has a limited solubilityin a solvent, and the impurity is insolublein that solvent.
Normally a solid material containing some of the desired compound is placed inside a thimble made from thick filter paper, which is loaded into the main chamber of the Soxhlet extractor. The Soxhlet extractor is placed onto a flask containing the extraction solvent. The Soxhlet is then equipped with a condenser. The solvent is heated to reflux. The solvent vapour travels up a distillation arm, and floods into the chamber housing the thimble of solid. The condenser ensures that any solvent vapour cools, and drips back down into the chamber housing the solid material.
After extraction the solvent is removed, typically by means of a rotary evaporator, yielding the extracted compound. The non-soluble portion of the extracted solid remains in the thimble, and is usually discarded.
B.ANTIMICROBIALSTUDIES
The methanol and ethanol extracts were screened for the zone of inhibition against bacterial and fungal strains by cup-plate method. Both the extracts were dissolved in methanol and ethanol respectively.
BACTERIAL STRAINS:
A.GRAM POSITIVE GENERA ARE:
A. STAPHYLOCOCCUS AUREUS
B. BACILLUS SUBTILIS
B.GRAM NEGATIVE GENERA ARE:
A. ESCHERICHIA COLI
B. KLEBSIELLA
FUNGAL STRAINS:
A.ASPERGILLUS NIGER
5.EXPERIMENTAL
1.PREPARATION OF METHANOL EXTRACT :
A fenugreek seed sample was collected.Dry fenugreek seed (25 g) was cleaned and ground into small pieces using a mortar and pestle. The extraction was carried out by soxhlet extraction method. The fine powder was packed in the tightly in the soxhlet extractor and methanol (250 ml) was used as a solvent for extraction. The process was carried out for 6hrs.The extract was filtered and re-extracted under the same conditions to ensure complete extraction. The obtained extract was evaporated to dryness under reduced pressure at 60?C to get a dried solid product and was stored in dried bottles for further analysis.
2. PREPARATION OF ETHANOL EXTRACT:
A fenugreek seed sample was collected.Dry fenugreek seed (25 g) was cleaned and ground into small pieces using a mortar and pestle. The extraction was carried out by soxhlet extraction method. The fine powder was packed in the tightly in the soxhlet extractor and ethanol (250 ml) was used as a solvent for extraction. The process was carried out for 6hrs.The extract was filtered and re-extracted under the same conditions to ensure complete extraction. The obtained extract was evaporated to dryness under reduced pressure at 60?C to get a dried solid product and was stored in dried bottles for further analysis.
1. ANTIMICROBIAL STUDIES
The methanol and ethanol extracts were screened for the zone of inhibition against bacterial and fungal strains by cup-plate method. Both the extracts were dissolved in methanol and ethanol respectively.
BACTERIAL STRAINS:
A.GRAM POSITIVE GENERA ARE:
1. STAPHYLOCOCCUS AUREUS
2. BACILLUS SUBTILIS
B.GRAM NEGATIVE GENERA ARE:
1. ESCHERICHIA COLI
2. KLEBSIELLA
FUNGAL STRAINS:
A. ASPERGILLUS NIGER
Test compounds were taken at a concentration of 100mg/ml. Nutrient agar media was used for culturing of bacteria and potato dextrose agar was used for the culturing of fungi.
COMPOUND NO 1:
Methanol extract of fenugreek
COMPOUND NO. 2:
Ethanol extract of fenugreek
INVITRO ANTI BACTERIAL STUDIES
a) Preparation of test inocula:
The clinical samples of the following strains (Bacillus subtilis, Staphylococcus aureus, Klebsiella, Escherichia coli) were obtained.
From the cultures obtained, using a sterilized Pasteur loop (3mm diameter) one loopful of each of the above mentioned bacterial cultures were transferred into a sterile nutrient agar slant by streaking. The slants were incubated for 24-48 hours at 37?C which showed sufficient growth for culturing in the media.
The above mentioned organisms inoculated in the test tube containing the media.
These test tubes were incubated at 37?C for 24 hours.
b) Preparation of drug solution:
The solid methanol and ethanol extracts were made into solution by dissolving in methanol and ethanol respectively. The concentration of the solution was 100mg/ml. A drug solution of standard drug of ampicillin at a concentration of 100µg/ml was prepared by dissolving in distilled water.
MEDIA USED FOR THE GROWTH OF THE BACTERIA:
NUTRIENT AGAR MEDIA
Constituents of Nutrient Agar Media:
1) MEAT EXTRACT - 3gm
2) PEPTONE - 5gm
3) SODIUM CHLORIDE - 8gm
4) AGAR - 15gm
5) DISTILLED WATER - 1000ml
The above constituents were put together, adjusted the PH to 7.4 and autoclaved at 121?C at 15lb pressure for 15mins and carry out the zone of inhibition method.
SCREENING OF ANTIBACTERIAL ACTIVITY OF THE EXTRACTS BY MODEL SYSTEMS:
ZONE OF INHIBITION METHOD:
For screening of antibacterial activity by cup-plate method, commercial samples of ampicillin were selected for their antibacterial activity by agar cup-plate method in nutrient agar medium.
Petridishes were filled to a depth of 4.5mm with a nutrient agar medium that has previously been inoculated with a suitable inoculation of a suitable test organism of B.subtillis, S.aureus, E.coli, Klebsiella individually. The petri dishes were allowed to cool for 1hr before use . With a small sterile borer of uniform size alluminium or stainless steel, holes were made in the medium for the standard drug (ampicillin) and for the test drugs (methanol and ethanol extracts of fenugreek).
Then 0.1ml of the solutions were introduced into the holes by means of a 1ml syringe delivery. The zone of inhibition was measured after incubation at 37?C for 24hrs.
INVITROANTI FUNGAL STUDIES
a) Preparation of test inocula:
The clinical samples of the strains of asperigillus were obtained.
From the culture obtained using a sterilized Pasteur loop (3mm in diameter) one loopful of the strain was transferred into the sterile Potato Dextrose Agar slant by streaking. The slants were incubated for24hrs at 37?C, which then showed sufficient growth for culturing in the broth.
The strains of Aspergillus were inoculated into the test tubes containing 10ml sterile potato dextrose agar medium. These test tubes were incubated at 37?C for 3days.
b) Preparation of drug solution:
The solid methanol and ethanol extracts were made into solution by dissolving in methanol and ethanol respectively. The concentration of the solution was 100mg/ml. A drug solution of standard drug of cotrimazole at a concentration of 100µg/ml was prepared by dissolving in distilled water.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MEDIA USED FOR THE GROWTH OF FUNGI WAS POTATO DEXTROSE AGAR MEDIA:
Constituents of PDA Media:
1) POTATO INFUSION -200gm
2) DEXTROSE -20gm
3) AGAR -20gm
4) DISTILLED WATER -1LT
The above constituents were put together, PH was adjusted to 5.4 and autoclaved at 121?C at 15lbs for 15mins and used for the sub culturing of aspergillus niger.
SCREENING OF ANTI FUNGAL ACTIVITY OF THE EXTRACTS BY MODEL SYSTEMS:
ZONE OF INHIBITION METHOD:
For screening of antifungal activity by cup-plate method, commercial samples of cotrimazole were selected for their antifungal activity by cup-plate method in potato dextrose agar medium.
Petridishes were filled to a depth of 4.5mm with a potato dextrose agar medium that has previously been inoculated with a suitable inoculation of a suitable test organism of Aspergillus niger individually. The petri dishes were allowed to cool for 1hr before use. With a small sterile borer of uniform size alluminium or stainless steel, holes were made in the medium for the standard drug (cotrimazole) and for the test drugs (methanol and ethanol extracts of fenugreek).
Then 0.1ml of the solutions were introduced into the holes by means of a 1ml syringe delivery. The zone of inhibition was measured after incubation at 37?C for 24hrs.
6. RESULT
EXTRACTION OF FENUGREEK SEEDS
Fenugreek seeds were extracted using soxhlet extraction method. Methanol and ethanol were used as solvents for the extraction process. The extraction was carried out for 6hours. The obtained filtrate was re-extracted under the same conditions to get the maximum yield. In general the extraction proceeded smoothly to get the expected yield. The extracts were evaporated under reduced pressure at 60?C to get dried solid products. Thus methanol and ethanol extracts of fenugreek were prepared and stored until further use.
ANTIMICROBIAL
Microorganisms are the concealed enemies to the mankind. They are small but cause a very profound damage in human body as well as other living organisms. The agents, which have the capacity to kill the microbes or arrest the multiplication, are called the antimicrobial agents or drugs. There are a lot of antimicrobial drugs of which some are discovered or established and some are hidden in the nature. Hence, the last decade witnessed an increase in the investigations on plants as a source of human disease management and more natural antimicrobials have driven scientists to investigate the effectiveness of inhibitory compounds such as extracts from plants. There are several reports of antibiotics resistance of human pathogens to available antibiotics. Biomolecules of plant origin appear to be one of the alternatives for the control of these antibiotic resistant human pathogens.
In the present work, the antibiotic potential of the methanol and ethanol extracts of the Fenugreek has been determined againstEscherichia coli, Bacillus, staphylococcus spp., and klebsiella. In this study, crude methanol extracts of Fenugreek were found to be effective in inhibiting the growth of E. coli, klebsiella , On the other hand, crude ethanol extract of Fenugreek showed antimicrobial activity against E. coli and Staphylococcus spp.,. The extract showed good antifungal activity against Aspergillus niger.
7.CONCLUSION
The extracts of Trigonella foenum were found to be effective antimicrobial agents sagainst human pathogens. This study paves the way for further attention and research to identify the active compounds responsible for the plant biological activity. Further studies should be undertaken to elucidate the exact mechanism of action by which extracts exert their antimicrobial effect.
RESULTS OF ANTI MICROBIAL STUDIES
Table:
|
ZONE OF INHIBITION IN MM |
|
|||||||
|
S.NO |
NAME OF THE DRUG |
BACILLUSSUBTILIS |
STAPHYLO COCCUS |
ESCHERICHIA COLI |
KLEBSIELLA |
ASPERGI ILLUS |
|
|
|
1. |
Methanol extract |
1.5 |
2 |
3 |
2.5 |
2 |
|
|
|
2. |
Ethanol extract |
2.5 |
4 |
2 |
1.5 |
3 |
|
|
|
3. |
Ampicillin |
5.5 |
6 |
7 |
5 |
_ |
|
|
|
4. |
Cotrimazole |
_ |
_ |
_ |
_ |
6.5 |
|
|
(FIGURE NO: 1)
GRAPHICAL REPRESENTATION OF ANTIMICROBIAL ACTIVITY
8. REFERENCES
1. E. N. Frankel, Food Chem., 57 (1996) 51
2. C. Lucia, P. Calogero, Z. Maurizio, C. Antonella,G. Silvia, S. Franco, T.Sabrina, and G. Luciano, J. Agric. Food Chem., 51 (2002) 927
3. H. L. Madsen and G. Bertelsen, Trends. Food SciTechnol., 6 (1995) 271.
4. H. L. Madsen, B.R. Nielsen, G. Bertelsen, and L.H. Skibsted, Food Chem., 57 (1996) 331.
5. P. K. Marja, I. H. Anu, J. V. Heikki, R. Jussi-Pekka, P. Kalevi, S. K. Tytti, and Marina, H. J.Agric. Food Chem., 47 (1999) 3954.
6. W. Zheng, and S.Y. Wang, J. Agric. Food Chem., 49 (2001) 5165.
7. F. J. Alarcon-Aguilara, R. Roman-Ramos, S. Perez-Gutierrez, A. Aguilar Contreras, C. C. Contreras-Weber and J. L. Flores-Saenz, J. Ethnopharmacol., 61 (1998) 101.
8. R. D. Sharma, A. Sarkar, and D. K. Hazra, Phytother. Res., 10 (1996) 332.
9. P. Ody, New York: Dorling Kindersley., 47 (1993)164.
10. P. Fleiss, Mothering. Summer (1988) 68.
11. C. Billaud, Sciences-des-ailments., 21 (2001) 3.
12. Y. Sauvaire, G. Ribes, J. C. Baccou and M. M.Loubatieres-Mariani, Lipids Mar., 26 (1991)191.
13. G. Ribes, Y. Sauvaire and C. D. Costa, Proc SocExp Biol Med., 182 (1986) 159.
14. E. Basch, C. Ulbricht, G. Kuo, P.Szapary and M.Smith, Altern Med Rev., 8 (2003) 20.
15. E. Miraldi, S. Ferri and V. Mostaghimi, J.Ethnopharmacol., 75 (2001) 77.
16. P. Khosla, D. D. Gupta, and R. K. Nagpal, Int. J.of Pharmacol., 27 (1995) 89. 17. M. M. Cowan, Clin. Microbiol. Rev.,12 (1999)564.
18. K. Shetty, Asia. Pac. J. Clin. Nutr., 21 (1997)79.
19. S. N. Lim, P.C.K. Cheung, V. E. C. Ooi and P.O. Ang, J. Agric. Food Chem., 50 (2002) 3862.
20. M. E. Prashani, T. P. Geun, D. L. Young, K.Sejae, J. Sang and L. Jehee, J. Food lipids., 12(2005) 34.
21. S. Iqbal, M. I. Bhanger and A. Farooq, FoodChem., 93 (2005) 265.
22. Y. U. Liangili, P. jonathan, H. Mary, W. Johnand H. Scott, J. Agric. Food Chem., 51 (2003)1566.
23. J. Zhishen, T. Mengcheng and W. Jianming,Food Chem., 64 (1999) 555.
24. R. Re, N. Pellegrini, A. Proteggente, A. Pannala,M. Yang, C. Rice-Evans Free Radic Biol. Med.,26 (1999) 1231.
25. S. Perumal and B. Klaus, J. Agric. Food Chem.,51 (2003) 2144
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









