 About Authors:
About Authors:
A.Tamil Selvan*, R.Suresh1, D.Benito Johnson2, R.Suresh Kumar3, R.Venkatanarayanan4, L.Sivakumar5
Department of Pharmacology
Teegala Ram Reddy College of Pharmacy, Meerpet, Hyderabad – 97
1-4RVS College of Pharmaceutical Sciences, Sulur, Coimbatore- 641402
5SKM Siddha and Ayurvedha Company (India) Limited, Erode - 638104.
*tamilselvanpharmacologist@gmail.com
ABSTRACT
In this study, polyherbal extract of Cycas circinalis, Nardostachys jatamansi and Artemisia absinthium ethanolic fraction was explored for its antidepressant property using Forced swim test (FST) and Tail suspension test (TST). Many of its individual constituents have been used for central nervous system (CNS) activities but no systematic work was carried on this combination. In this study its effect on depression was explored in rats. For this purpose the plants part were extracted by successive solvent extraction by Soxhylation. Ethanolic extract was chosen for the pharmacological evaluation, based upon the phytochemical and instrumental analysis. The ethanolic extract was subjected to FT-IR analysis for finding the possible number and nature of function groups present in it. Also the extract was analysed by HPLC for the number possible phytocompounds/phytoconstituents present, which may directly or indirectly involve in the brain neurochemical activity. Acute and Subacute toxicity study revealed the dose upto 2000mg/kg the extract had not toxic symptoms and no mortality. The therapeutic dose was found to be 200mg/kg and there was no toxic damage to liver and kidney observed in subacute toxicity study. The ethanolic extract of the polyherbal combination exhibited significant (P<0.001) antidepressant activity as indicated by its ability to decrease swim stress and tail suspension induced immobility time in rats as compared with that standard Fluoxetine. As well as restoring biogenic amines to normal level that was altered by the swim stress and tail suspension test in whole rat brain assay by HPTLC method. The result indicates this polyherbal combination can be a potential candidate for .managing depression. However further studies are required to confirm the exact therapeutic efficacy.
Reference Id: PHARMATUTOR-ART-1515
INTRODUCTION
The Indian system of medicine Ayurveda, Siddha, Unani and Homeopathic system predominantly use plant based raw materials and most of their preparations and formulations and formulations. Herbal medicines are becoming more and more popular now a days. Demand for medicinal plant is increasing in both developed and developing countries due to growing recognition of natural products and sometimes the only source of health care available to the poor1. Mental health problems currently are said to constitute about 8% of the global burden of disease and more than 15% of adults in developing societies are estimated to suffer from mental illness. Depression and anxiety are the two most frequent mental disorders. More than 20% of the adult population suffers from these conditions at some times during their life time. The World Health Organisation predicts that depression will become the second leading cause of premature death or disability worldwide by the year 2020. Since, ancient time the herbal medicines are effective in the treatment of various ailments. Many plants have folklore claim in the treatment of several dreadful diseases but they are not significantly exploited and/or improperly used. Therefore these plant drugs deserve detailed studies in the light of modern medicine9.
The plants Cycas circinalis, Nardostachys jatamansi and Artemisia absinthium were traditionally used to treat various ailments and mental disorders. Cycas circinalis pacifies vata, pitta, pain, inflammation, swelling, flatulence, vomiting and general debility. The pollen is narcotic. The bark and the seeds are ground to paste with oil and used as a poultice on sores and swellings6. Artemisia absinthium is used as nervine tonic, particularly helpful against the falling sickness and for flatulence. A dried, encapsulated for of plant is used as anthelmintic10. Nardostachys jatamansi is useful for urine related problems and maintaining the circulatory system. It is one of the best herbs for treating epilepsy. Used as an adjunct in the treatment of sexual debility and impotence. Jatamansi is one of the brain tonic useful in memory. Internally, the herb is useful in various diseases. Also improves digestion, effective against vertigo, seizures etc8.
The literature review, ethnobotanical survey and the traditional usage of these herbs reveals the treatment of the various diseases from the time immemorial. Based upon these views the present combination of polyherbal was chosen for antidepressant evaluation. No detailed study was conducted for its central nervous system effect on this combination regarding its effect of CNS. Hence the present study is aimed to evaluate antidepressant profile of these polyherbal.
MATERIAL AND METHOD
Coarsely powdered materials of the plants Cycas circinalis (flower),Artemisia absinthium (whole plant) and Nardostachys jatamansi (roots) were collected from SKM Siddha and Ayurvedha Company (India) Limited, Erode. The powder was subjected to various studies for which the materials and methods presented below.
EXTRACTION OF PLANT MATERIALS3
Equal amount of the weighed coarse powders of polyherbal combination was extracted by successive solvent extraction by Soxhlet apparatus using various solvents. About 500gm of coarse powder was extracted with 2.5 litre of petroleum ether (60-80°C) by continuous hot percolation using Soxhlet apparatus. The extraction was continued up to 24hours.After completion, the petroleum ether was extract was filtered and solvent removed by distillation under reduced pressure. Then the obtained residue was stored in a dessicator. Marc obtained from the above extract was dried and extracted with 2.5litre of ethanol. Then it was filtered and stored in dessicator. From the weight of each extractive residue, the extractive values were calculated in percentage. All the above extracts were used for identification of constituents by preliminary phytochemical tests.
PRELIMINARY PHYTOCHEMICAL EVALUATION
The various extracts of combination were subjected to chemical tests for identification of plant constituents by chemical tests for the presence of the phytocompounds like alkaloids, carbohydrates and glycosides, phytosterols, fixed oils and fats, saponins, tannins and phenolic compounds, proteins and free amino acids, gums and mucilage, flavonoids and lignin.
INSTRUMENTAL ANALYSIS
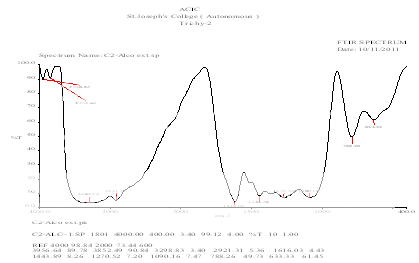
FT-IR - The ethanolic extract which contain more phytocontituents were chosen for the FT-IR analysis. The FT-IR analysis of the plant extracts in KBr pellets by using FT-IR spectroscopy Shimadzu, IR affinity 1, Japan) at moderate scanning speed between 4000 - 400 cm-1. The peak values (wave numbers) and the possibility of functional group were shown in Table-1.
HPTLC- Chromatography was performed on silica gel F254 HPTLC pre-coated plates. Samples were applied on the plates as band of 7mm width using a Camag Linomat V sample applicator at the distance of 14mm from the edge of the plates. The mobile phase was constituted of ethyl acetate-acetic acid-formic acid-water 100:11:11:27 (V/V/V/V). After development, plates were dried and derivatised in NP-PEG reagent. The finger prints were evaluated at 366nm in fluorescence mode with WinCats and VideoScan software. Ethanolic extract of the combination was taken for the analysis. Peaks, Rf values, Peak height and Peak area were given in Table - 2.
TOXICITY STUDY
Toxicokinetics studies (generation of pharmacokinetic data either as an integral component of non-clinical toxicity studies or in specially designed studies) should be conducted to assess the systemic exposure achieved in animals and its relationship to dose levels and duration of treatment. Toxicity testing is of paramount importance while screening drugs4. The experimental protocol was approved by Committee for the Purpose of Control and Supervision of Experiments on Animals and Institutional Animal Ethics Committee (IAEC) Registration number (1012/c/06/CPCSEA) of RVS College of Pharmaceutical Sciences, Sulur,Coimbatore-641402.The procedure was followed by using OECD guidelines (Organization of Economic Corporation and Development). The method used defined doses (2000 mg/kg, p.o.) and results allow a substance to be ranked and classified according to the Globally Harmonized System (GHS) for classification of chemical which cause acute toxicity (OECD 423 Acute class method). Subacute toxicity study was done by divided the dose in the range of 1/10th, 1/20th and 1/5th. Based upon that the therapeutic dose was found and fixed.
PHARMACOLOGICAL EVALUATION
The present study is designed with the following models in order to asses the antidepressant activity of polyherbal combination. Albino wistar rats of either sex weighing 160-180gm each were housed at 24±2°C with 12:12 hour light and dark cycle. They had free access to food and water ad libitum. The animals were acclimatized for a period of 7 days before the study. All the experiments were carried out between 10.00 to 16.00 hour at ambient temperature. The animals were drawn at random for test and control groups.
FORCED SWIMMING TEST7
The FST is the most widely used pharmacological model for assessing antidepressant activity. The development of immobility when the rodents are placed in an inescapable cylinder of water reflects the cessation of persistent escape-directed behaviour. The test was performed according to a modification suggested of the traditional method described by Porsolt et al. The apparatus consisted of a transparent Plexiglas cylinder (50 cm high×20 cm wide) filled to a 30 cm depth with water at room temperature. Animals were divided into 3 groups of each consisting 6 animals and were administered 1 h prior to the beginning of swimming session in case of test compounds and 0.5 h prior in case of standard drugs. The study was initiated 24 hrs after the pre-test session which consisted of allowing the animals to swim for 15 min. At the end of this session animals were returned to their respective home cages after drying them in a heated enclosure. The rats were judged to be immobile when it is floated in an upright position, and made only small movements to keep its head above water. The duration of immobility was recorded during the last 5 min of the 6 min testing session.
Group-I: Animals received distilled water (1 ml/kg, p.o.),
Group-II: Animals received Fluoxetine (20 mg/kg, p.o.) in distilled water,
Group-III: Animals received alcoholic extract of C2 (200 mg/kg, p.o.) in distilled water.
TAIL SUSPENSION TEST2
TST is a simple, rapid and reliable method to screen antidepressants and other class of psychotropics. This method is based on the observation that a mouse suspended by the tail shows alternate agitation and immobility which is indicative of a state of depression. TST induced immobility is reduced by a large number of clinically active and a typical antidepressants. Device used for this consists of metallic gallows which were connected to a nylon catheter (d=1.5 mm, l=150 mm) with a hook attached to its extremity. The distance between the floor of the device and the hook was 350 mm. The mouse was hung in the hook by an adhesive tape placed 20 mm from the extremity of its tail, the mouse was 150 mm away from the nearest object. Mice were suspended individually by the tail by securing the tail to the shelf by adhesive tape placed approximately 2 cm away from the tip of the tail. And the total immobility time was noted. Results shown in Table – 3.
Group-I: Animals received distilled water (1 ml/kg, p.o.), Group-II: Animals received Fluoxetine (20 mg/kg, i.p.) in distilled water, Group-III: Animals received alcoholic extract of C2 (200 mg/kg, p.o.) in distilled water.
ESTIMATION OF BIOGENIC AMINES2
For antidepressant action the animals screened by forced swimming test were chosen and their neurotransmitters dopamine, nor-adrenaline and serotonin (5-HT) were quantified by HPLC method by electrochemical detection. At the end of FST the experimental period the animals were sacrificed by cervical dislocation, whole brain was dissected out and the sub-cortical region (including the striatum) was separated on an ice packing and homogenized in ice-cold buffer solution (0.85 ml of hydrochloric acid in one litre n-butanol, pH-3.96) for 1 min in a cool environment (00C).
Then, the homogenate was centrifuged at 4500 rpm for 30 min, supernatant was separated and ultrafiltered through a 0.22µm filter paper. The clear ultrafiltrate was used for quantification of DA, NA and 5-HT using HPLC method. Results shown in Table – 4.
STATISTICAL ANALYSIS
Results were represented as mean+SEM. Data was analysed using a statistical package (Graph pad prism version 3.00 to Windows, Graph pad software, San Diego, California,(USA).Comparison between groups were made using one-way analysis of variance (ANOVA) a post-hoc comparisons were performed using Tukey-multiple comparison test.
RESULTS
The extractive value indicates the yield of the extract obtained from the air dried plant powder by successive solvent extraction by Soxhlet extraction. Their percentage yield shows the solubility of the active principles in the organic solvents used based upon the polarity nature. They are then identified and confirmed by the preliminary phytochemical evaluation. The presence of phytoconstituents like alkaloids, flavonoids, carbohydrates, tannins, phytosterols, proteins & amino acids, gums & mucilage and resins are responsible for the typical pharmacological effects.
FT-IR ANALYSIS- The possible presences of the functional groups were obtained from the FT-IR spectrum of the extracts and they were shown in table. HPTLC ANALYSIS - The finger print of the constituents present in samples was recorded using Camag and TLC visualiser and WinCats Software. The various compounds isolated at various peaks, Rf value, peak height and peak area were identified and presented in the table.
TOXICITY EVALUATION
The LD50 determination was done in mice by OECD guideline 423 and LD50 of extract was determined (infinity). In this study there was no toxicity/death were observed at the dose of 2000 mg/kg body weight in animals. The acute toxicity study showed that at 200mg/kg dose the extracts are safe for consumption and for medicinal uses. The therapeutic dose of the drug was considered as 1/10th of the LD50 value. Hence, the therapeutic dose used for recording biological response was 200 mg/kg, p.o for the extracts. Subacute toxicity study shows no signs of toxicity or mortality. Hence the extract was so safe for chronic therapy too.
ANTIDEPRESSANT ACTIVITY
Ethanolic extract significantly decreased the immobility time of animals subjected to forced swimming. The ethanolic extract at the dose of 200 mg/kg produced significant (p<0.001) antidepressant effect in forced swimming test, as evident from the reduction in the immobility time and the effect was comparable to the standard drug Fluoxetine. It was expected that immobility occurs in this test will reflect a state of behavioural despair or unable to adapt the stress as seen in human. The basic concept of forced swimming test was animal will get immobile posture when subjected to the short-term or inescapable stress. The extract at the dose of 200 mg/kg administered to mice 1 hour before they were subjected to tail suspension test showed significant antiimmobility action when compared with Fluoxetine (20 mg/kg). The ethanolic extract significantly decreased the immobility time of animals subjected to tail suspension. The ethanolic extract at the dose of 200 mg/kg produced significant (p<0.001) antidepressant effect in tail suspension test, as evident from the reduction in the immobility time and the effect was comparable to the standard drug Fluoxetine.
ESTIMATION OF BIOGENIC AMINES
The increased ambulatory indicates a stimulant effect and prompted us to study it further using other paradigms of depression like tail suspension and forced swimming. The immobility exhibited by test animals in these models is an indicative of a behavioural despairness which reflects a state of depression. The ethanolic extract combination significantly reduced the immobility time. Thus, showed the potential antidepressant nature of the extract. Hence it is necessary to estimate all three neurotransmitters in animal depressed by forced swimming. Untreated controls showed decreased levels of noradrenaline, 5-HT and dopamine indicating a state of depression. On the other hand extract treated animals exhibited increased levels these biogenic amines. The various types of stressors induced hormonal alternations in experimental animals which were reminiscent of those observed in depressed patients. Forced swimming in animal used as a swim stressor to the study the action of antidepressant drugs on endocrine systems. The extract treated animals showed significant (p<0.001) increase in noradrenaline, dopamine and 5-HT levels compared with the standard drug Fluoxetine. Reuptake of biogenic amines into their respective neurones and thus potentiate them. They, however, differ markedly in their selectivity and potency for different amines. Initially the presynaptic α2 and 5-HT1auto receptors are activated by the increased amount of NA/5-HT1in the synaptic cleft resulting in decreased firing of locus ceruleus (noradrenergic) and raphe (serotonergic) neurones. Thus, uptake blockade appears to initiate a series of time-dependent changes that culminate in antidepressant activity.
DISCUSSION
The presence of the functional groups directly or indirectly involved in the pharmacological actions of the selective Indian medicinal plants. The functional groups of the phytoconstituents present in the extract may modify the CNS actions which lead to the selective biological response in the preclinical animal models. Functional groups of the phytochemicals similar to the functional group of the brain biochemical act according to the SAR and there by produced the required neuropharmacological response. The number of peaks at various Rf values shows the different compounds present in the crude extracts. They were isolated at particular finger print region with their respective peak areas and peak height. The extract contains many peaks confirms many number of active constituents present in it. Those extracts were chosen for the preclinical evaluation. Those phytocompounds were responsible for the specified pharmacological actions in the brain which reveals the extract for antidepressant activities.The cumulative toxicity of a substance on target organs or physiological and metabolic effects at low dose on prolonged exposure can be studied by sub acute toxicity. The results from sub acute toxicity studies can provide valuable information, which helps in selecting dose levels. The long term safety level of a compound can be predicted from acute or shorter than subacute studies. Sub acute toxicity studies are generally carried out for a period ranging from a few days to three months. The present investigation demonstrates that at doses consumed in the extracts may be considered as relatively safe, as it did not cause either any lethality or changes in the general behaviour in both acute and subacute toxicity studies in rats. Studies of this type were needed before a phytotherapeutic agent can be generally recommended for use.
The present study showed antidepressant like effects in animal models of depression. The precise mechanism might be increased the concentration of neurotransmitters and serotonin transmission. Some studies suggested that 5-HT modulation might be producing anti-depressant effects independent of changes to synaptic transmission. Increase in serotonin level through blockade of 5-HT receptors could possibly explain the overall antidepressant like effects of extracts. Fluoxetine a selective serotonergic reuptake inhibitor facilitate serotonergic neurotransmission. Since 5-HT implicated in etiology of depression, the positive effects of these drugs in tail suspension test seems to be due to increased availability of the neurotransmitters at the post synaptic sites. Studies suggested that involvement of ethanolic extract might have increased monoamines level at post synaptic sites. Decreased dopamine transmission was also linked with depression and it may be demonstrated that blockade of dopamine receptor by extracts increased duration of immobility. Hence the extract might be served as a potential resource for natural psychotherapeutic agents against stress related disorders. Inhibition of dopamine, norepinephrine and 5-HT uptake to produce antidepressant action. Reuptake inhibitors results in increased concentration of the amines in the synaptic cleft in CNS and periphery also.
SUMMARY AND CONCLUSION
Anxiety, depression and mental health problems in general and senile neurological disorders in particular, are widely prevalent in modern fast-paced life with a multitude of stressful conditions. Depression was affecting one-eighth of the total population of the world and become a very important area of research in psychopharmacology during this decade. In recent years, various types of herbal medicines have been used as antidepressant drugs in different parts of the world. This amounts to 12.3% of the global burden of disease, and will rise to 15% by 2020 (Reynolds, 2003). In the search for new therapeutic products for the treatment of neurological disorders, medicinal plant research, world wide, has progressed constantly, demonstrating effectiveness of different plant species in a variety of animal models (Zhang, 2004). Keeping these facts in mind the present investigation was undertaken to investigate the putative antidepressant activity of the selective Indian medicinal plants. The beneficial medicinal effects of plant materials typically result from the combinations of secondary metabolites present in the plant, through additive or synergistic action of several chemical compounds acting at single or multiple target sites associated with a physiological process (Briskin 2000). Findings in this study justify the use of selected Indian medicinal plants in traditional medicine while also suggesting a potential usefulness in the treatment of depression.
ACKNOWLEDGEMENT
I thank Dr.R.Venkatanarayanan M.Pharm., Ph.D Principal, RVS College of Pharmaceutical Sciences for his valuable, worthable, whole hearted support during the course of this research work. I would like to thank Dr.L.Sivakumar (Managing Director), Mr.M.Senthil Kumar (Manager - Quality Assurance), Mr.M.Visvanath (Manager - Quality Control), Mr.K.M.Palanisamy, Mr.C.Balakrishnan and Mr.Ganaprakasam (Chemists), SKM Siddha and Ayurvedha Company (India) Limited, Erode for their continuous support, dedicated advice and strong support in completing this work successfully.
TABLE – 1
FT-IR ANALYSIS OF ETHANOLIC EXTRACT
|
S.No |
Peak value nm-1 |
Functional group |
|
1 |
3298 |
N-H Stretching |
|
2 |
2921 |
Alkyl C-H stretching |
|
3 |
1616 |
Aromatic C=C stretching |
|
4 |
1443 |
Aromatic C-H bending(asymmetrical) |
|
5 |
1270 |
C-O stretching |
|
6 |
788,633 |
Aromatic mono substitution |
TABLE – 2
HPTLC ANALYSIS OF ETHANOLIC EXTRACT
|
Peaks |
Rf |
Peak Height |
Peak Area |
|
Peak 1 |
0.13 |
538.94 |
37296.37 |
|
Peak 2 |
0.33 |
262.95 |
11270.11 |
|
Peak 3 |
0.42 |
Not detected |
Not detected |
|
Peak 4 |
0.63 |
256.85 |
8159.92 |
|
Peak 5 |
0.68 |
Not detected |
Not detected |
|
Peak 6 |
0.95 |
100.58 |
1585.55 |
|
Peak 7 |
1.04 |
124.06 |
4737.87 |
|
Peak 8 |
1.15 |
164.92 |
8328.72 |
|
Peak 9 |
1.32 |
77.70 |
2626.51 |
|
Peak 10 |
Not detected |
Not detected |
Not detected |
|
Peak 11 |
1.84 |
436.67 |
26592.11 |
FT-IR SPECTRUM OF ETHANOLIC EXTRACT
HPTLC CHROMATOGRAM OF ETHANOLIC EXTRACT
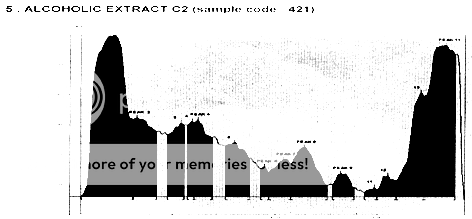
TABLE – 3
ANTIDEPRESSANT ACTIVITY BY TAIL SUSPENSION AND FORCED SWIMMING TEST
|
S.No |
Treatment |
Immobility(s) TST |
Duration of FST(s) |
|
1 |
Control |
117.00±1.238 |
81.5±0.671 |
|
2 |
Fluoxetine (20mg/kg, p.o.) |
61.5±0.619* |
34.5±3.243* |
|
3 |
Ethanolic extract (200mg/kg, p.o.) |
83.33±1.874* |
61.00±0.073* |
n=6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.001)
TABLE – 4
ESTIMATION OF BIOGENIC AMINES IN ANTIDEPRESSANT STATE
|
S.No |
Treatment |
Norepinephrine |
Dopamine |
5-HT(ng/gm) |
|
1 |
Control |
2419±281.1 |
2969±296.7 |
3955±425.0 |
|
2 |
Fluoxetine (20mg/kg, p.o.) |
2930±152.2* |
2512±173.2* |
3417±361.4* |
|
4 |
Ethanolic extract (200mg/kg, p.o.) |
2840±142 |
3576±224.1 |
4975±343.6 |
n=6, Values are mean ± SEM. Differences were assessed statistically using one-way ANOVA followed by Tukey’s test. *(p<0.001)
REFERENCES
1) Edwin, E et al (2005). Herbal medicines: the ups and down. The Ind. Pharmacist. 6, 38?44.
2) K.N.Najappa et al Pharmacological and Neurobiochemical evidence for Antidepressant like effect of Sumind in animals, The Internet Journal of Nutrition and Wellness, ISSN: 1937-8297.
3) Kokate, CK (1991). Practical Pharmacognosy. 5th ed, Vallabh Prakasham Delhi; 107-121.
4) Kulkarni, SK (1999). Hand book of Experimental Pharmacology, Vallabh Prakashan Delhi; 3:29-42.
5) Mental illness. National Association for Mental Health. Available on www.mind .org.uk. Dated on 04?8?2008.
6) Newberne, PM (1976) Biologic effects of plant toxins and aflatoxins in rats. J Natl Cancer Inst. Mar; 56(3):551-5.
7) Porsolt, RD (1977). Behavioral despair in mice: A primary screening test for antidepressants. Archives Internationales De Pharmacodynamie et de Therapie 229, 327-336.
8) Shah, J et al (2011) investigation of neuropsychopharmacological effects of a polyherbal formulation on the learning and memory process in rats. J Young Pharm. Apr; 3(2):119-24.
9) Tripathi, KD (2003). Essentials of Medical Pharmacology. Jaypee Brothers Publishers, New Delhi; 399-402.
10) Y?ld?z, K (2011) Antiparasitic efficiency of Artemisia absinthium on Toxocara cati in naturally infected cats. Turkiye Parazitol Derg; 35(1):10-4.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









