{ DOWNLOAD AS PDF }
ABOUT AUHTOR
G.Swapna
Department of pharmaceutical Analysis & Quality Assurance,
Nirmala college of pharmacy, Guntur,
AP, India
swapna.goday.gs@gmail.com
ABSTRACT
To make drugs serve their purpose various chemical and instrumental methods were developed and regular intervals which are involved in the estimation of drugs. A photoelectric flame photometer is a device used in inorganic chemical analysis to determine the concentration of certain metal ions, among them sodium, potassium, lithium, and calcium. Group 1 and Group 2 metals are quite sensitive to Flame Photometry due to their low excitation energies [1].These pharmaceuticals would serve their intent only if they are free from impurities and are administered at appropriate amounts [2] . These pharmaceuticals develop impurities at various stages of their development, transportation and storage which makes the pharmaceuticals risky to be administered thus they may be detected and quantified. For this analytical instrumentation and methods play important role. This review highlights a variety of analytical techniques for analysis of inorganic elements. Analytical techniques for analysis of inorganic elements. The most commonly used techniques for the determination of inorganic elements is atomic spectroscopy the different branches of atomic absorption spectroscopy are(1) flame photometry or flame atomic emission spectrometry. (2) atomic absorption spectrophotometer, (aas). (3) inductively coupled plasma-atomic emission spectrometry (icp-aes).(4) uv - visible spectrophotometer.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2467
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 2 Received On: 16/08/2016; Accepted On: 14/11/2016; Published On: 01/02/2017 How to cite this article: Swapna G; Analytical techniques for analysis Of Inorganic Elements – A Review; PharmaTutor; 2017; 5(2); 39-45 |
INTRODUCTION
The most commonly used techniques for the determination of inorganic elements is ATOMIC SPECTROSCOPY The different branches of atomic absorption spectroscopy are
(1) Flame photometry or flame atomic emission spectrometry
(2) Atomic absorption spectrophotometry, (AAS)
(3) Inductively coupled plasma-atomic emission spectrometry (ICP-AES).
Photoelectric flame photometry
Principle:
Photoelectric flame photometry, a branch of atomic spectroscopy is used for inorganic chemical analysis .The basis of flame photometric working is that metals are dissociated due to the thermal energy provided by the flame source. Due to this thermal excitation, some of the atoms are excited to a higher energy level where they are not stable. The subsequent loss of energy results movement of excited atoms to the low energy ground state with emission of some radiations, which can be visualized in the visible region of the spectrum. The absorbance of light due to the electrons excitation can be measured by using the direct absorption techniques while the emitting radiation intensity is measured using the emission techniques.
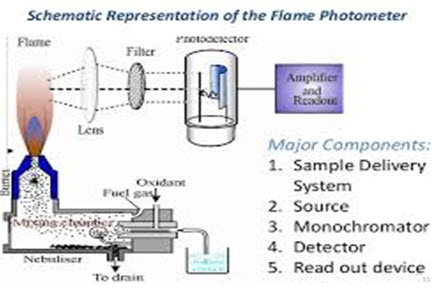
Figure 1. flame photometer
Pressure regulator
1 A 10 lb/in2 pressure regulator for fuel and a 20 lb/in2 pressure regulator for the air supply must be installed in line from gas cylinders to burners.
Source of flame
A burner that provides flame and can be maintained in a constant form and at a constant temperature.
Total consumption burner: In this sample is aspirated through a capillary by the high pressure of fuel &oxidant & burnt at the tip of the burner.
Laminar flow burner: In this ,the sample solution,fuel,oxidant are mixed before they reach the burner tip. Only few droplets of uniform size reaches the flame& remaining are drained out.
Nebuliser and mixing chamber : Helps to transport the homogeneous solution of the substance into the flame at a steady rate.
Optical system (optical filter):
The optical system comprises three parts: convex mirror, lens and filter. The convex mirror helps to transmit light emitted from the atoms and focus the emissions to the lens. The convex lens help to focus the light on a point called slit. The reflections from the mirror pass through the slit and reach the filters. This will isolate the wavelength to be measured from that of any other extraneous emissions. Hence it acts as interference type color filters.
Photo detector:
Detect the emitted light and measure the intensity of radiation emitted by the flame. That is, the emitted radiation is converted to an electrical signal with the help of photo detector. The produced electrical signals are directly proportional to the intensity of light.
Mechanism of working Nebulisation:
The solution of the substance to be analyzed is first aspirated into the burner, which is then dispersed into the flame as fine spray particles. A brief overview of the process:
1. The solvent is first evaporated leaving fine divided solid particles.
2. This solid particles move towards the flame, where the gaseous atoms and ions are produced.
3. The ions absorb the energy from the flame and excited to high energy levels.
4. When the atoms return to the ground state radiation of the characteristic element is emitted.
5. The intensity of emitted light is related to the concentration of the element.
The various processes in the flame are discussed below:
• Desolvation: The metal particles in the flame are dehydrated by the flame and hence the solvent is evaporated.
• Vapourisation: The metal particles in the sample are dehydrated. This also led to the evaporation of the solvent.
• Atomization: Reduction of metal ions in the solvent to metal atoms by the flame heat.
• Excitation: The electrostatic force of attraction between the electrons and nucleus of the atom helps them to absorb a particular amount of energy. The atoms then jump to the exited energy state.
• Emission process: Since the higher energy state is unstable the atoms jump back to the stable low energy state with the emission of energy in the form of radiation of characteristic wavelength, which is measured by the photo detector.
APPLICATIONS
• Flame photometer has both quantitative and qualitative applications
• Flame photometer with monochromators emits radiations of characteristic wavelengths which help to detect the presence of a particular metal in the sample.
• This help to determine the availability of alkali and alkaline earth metals which are critical for soil cultivation.
• In agriculture, the fertilizer requirement of the soil is analyzed by flame test analysis of the soil.
• In clinical field, Na+ and K+ ions in body fluids, muscles and heart can be determined by diluting the blood serum and aspiration into the flame.
• Analysis of soft drinks, fruit juices and alcoholic beverages can also be analyzed by using flame photometry.
ATOMIC ABSORPTION SPECTROCOPY
The process by which unexcited atoms in a flame, furnace or plasma absorb characteristic radiation from a source and attenuate the radiant power of the source.
PRINCIPLE
Atomic-absorption spectroscopy[4]quantifies the absorption of ground state atoms in the gaseous state . The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. The analyte concentration is determined from the amount of absorption.
INSTRUMENTATION
1. A light source ( usually a hollow cathode lamp )
2. An atom cell ( atomizer)
3 . A monochromator
4 . A detector , and
5. Read out device
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Figure 2. Atomic absorption spectroscopy
1.Light Source:
The light source is usually a hollow cathode lamp of the element that is being measured . It contains a tungsten anode and a hollow cylindrical cathode made of the element to be determined. These are sealed in a glass tube filled with an inert gas (neon or argon) . Each element has its own unique lamp which must be used for that analysis .

Figure 3. light source
Applying a potential difference between the anode and the cathode leads to the ionization of some gas atoms . These gaseous ions bombard the cathode and eject metal atoms from the cathode in a process called sputtering. Some sputtered atoms are in excited states and emit radiation characteristic of the metal as they fall back to the ground state . The shape of the cathode which is hollow cylindrical concentrates the emitted radiation into a beam which passes through a quartz window all the way to the vaporized sample.
2 . Atomizer
Elements to be analyzed needs to be in atomic sate. Atomization is separation of particles into individual molecules and breaking molecules into atoms .This is done by exposing the analyte to high temperatures in a flame or graphite furnace .The role of the atom cell is to primarily dissolvate a liquid sample and then the solid particles are vaporized into their free gaseous ground state form . In this form atoms will be available to absorb radiation emitted from the light source and thus generate a measurable signal proportional to concentration
3. Monochromators This is a very important part in an AA spectrometer. A monochromator is used to select the specific wavelength of light which is absorbed by the sample, and to exclude other wavelengths. The selection of the specific light allows the determination of the selected element in the presence of others
4 .Detector The light selected by the monochromator is directed onto a detector that is typically a photomultiplier tube , whose function is to convert the light signal into an electrical signal proportional to the light intensity.
5. Read out Device The processing of electrical signal is fulfilled by a signal amplifier . The signal could be displayed for readout , or further fed into a data station for printout by the requested format.
AAS APPLICATIONS:
Clinical analysis : Analyzing metals in biological fluids such as blood and urine.
Environmental analysis : Monitoring our environment finding out the levels of various elements in rivers, seawater, drinking water, air, and petrol.
Pharmaceuticals. In some pharmaceuticalmanufacturing processes, minute quantities of a catalyst used in the process (usually a metal) aresometimes present in the final product. By using AAS the amount of catalyst present can be determined.
Industry : Many raw materials are examined and AAS is widely used to check that the major elements are present and that toxic impurities are lower than specified – e g in concrete, where calcium is a major constituent, the lead level should be low because it is toxic.
Mining: By using AAS the amount of metals such as gold in rocks can be determined to see whether it is worth mining the rocks to extract the gold .Trace elements in food analysis Trace element analysis of cosmetics ,Trace element analysis of hair.
CALCIUM FLAME COPPER FLAME POTASSIUM FLAME

Figure 4. flame color of elements
Figure 5. elements detected by atomic absoption are in pink colour boxes
Inductively coupled plasma-atomic emission spectrometry (ICP-AES)
A plasma is hot and electrically conducting gaseous mixture containing enough cations and electrons (though the plasma has a neutral charge overall) to maintain the conductance.
Principle:
Inductively Coupled Plasma Optical Emission Spectroscopy(ICP-OES) [3]is the measurement of the light emitted by the elements in a sample introduced into an ICP source
The ICP is an argon-based, radio frequency plasma & input rf frequency is either 27 or 40 MHz at powers from 1 to 2 kW. The temperature in the central analyte channel ranges from about 6000 to 8000° K.
At these temperatures most elements are largely atomized and ionized, these emit characteristic radiation.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
INSTRUMENTATION [4]
• inductively coupled plasma source
• Nebulizer
• Pumps
• Spray chambers
• Torch
• Radio frequency generators
• Transfer optics
• Wavelength dispersive devices
• Detector (photomultiplier; PMT)
• Data collection
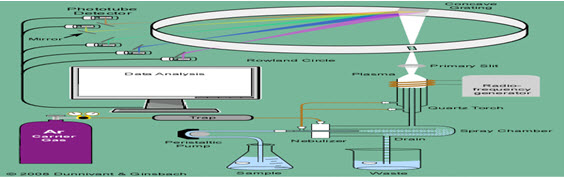
Figure 5. Inductively coupled plasma-atomic emission spectrometry (ICP-AES)
Sample introduction
NEBULIZER
the sample solution is acidified up to 2-3% in HNO3 to prevent adsorption of metals onto polypropylene sample bottle or onto instrument tubing or glassware prior to introduction into the plasma. Converts liquid sample into aerosol & is transported into plasma which excites the atomic species in the aerosol.
Two forces to break up this liquid
1. Pneumatic forces
2. Ultrasonic mechanical forces
PUMPS [5] Peristaltic pumps are almost exclusively the pumps of choice There is a special tubing used in it where the sample is passed through it This is for the prevention of contamination of direct contact with the solution
SPRAY CHAMBERS Once the sample aerosol is created by nebulizer, it is transported to the torch & injected into the plasma[6]. A spray chamber is placed between nebulizer and torch.The primary function is to remove large particles from the aerosol.Secondary purpose is to smooth out pulses that occur during nebulization While smaller particles travel with the Ar flow and enter the torch. Evaporation, atomization, and excitations/ionizations occur in the plasma at temperatures reaching 10 000 K.
TORCH There are 3 tubes for argon flow & aerosol injection.The spacing between 2 outer tubes is narrow, so that gas emerges at high velocity The purpose of the torch is to (1) evaporate the solvent (usually water) from the analyte salts,(2) atomize the atoms in the salt (break the ionic bonds and form gaseous state atoms),(3) excite or ionize the atoms. The gas flow that carries the sample aerosol is injected into the plasma through central tube or injectorTorches are of 2 namely Mountable & Demountable

Figure 6. torch
RADIO FREQUENCY GENERATORS It is the device that provides the power for the generation & sustainment of the plasma discharge power (700 to 1500 watts) is transferred to plasma gas through a load coil surrounding the top of the torch.2 types namely 1.Crystal controlled generators 2.Free running generators.
WAVE LENGTH DISPERSIVE DEVICES physical dispersion- diffraction grating.Prisms ,filters,interferometers, Echelle grating most commonly used.The Rowland system utilizes a concave Echellette-style grating monochromator .monochromatic radiation is composed primary of wavelenths representative of the light emitted by a particular elemental or molecular species just like musical instruments which produce harmonics of sounds.Multi element analysis is possible with conventional polychromators where each slit aligned to allow a specific wavelength of radiation to pass to a detector
DETECTORS Once the proper emission line has been isolated by the spectrometer[7] detected measures the intensity of the emission, By far the most widely used detector is Photo multiplier tube It is a vacuum tube contains photo cathode that ejects electrons when it is struck by light. These ejected electrons are accelerated towards dynode which ejects 2 to 5 secondary electrons for every electron which strikes at surface. the secondary electrons[8] strikes another dynode ejecting more electrons causing multiplicative effect.The final step is the collection of secondary electrons from the last dynode by the cathode.
Conclusion
Flame photometry is crude but cheap compared to Atomic Absorption spectrometer. Atomic Absorption spectrometer can be used to determine over 70 different elements in solution or directly in solid samples used in pharmacology, biophysics and toxicology research. Atomic absorption spectrometry has many uses in different areas of chemistry such as clinical analysis of metals in biological fluids and tissues such as whole blood, plasma, urine, saliva, brain tissue, liver, muscle tissue, semen, in some pharmaceutical manufacturing processes, minute quantities of a catalyst that remain in the final drug product, and analyzing water for its metal content.
REFERENCES
1. https://en.wikipedia.org/wiki/Photoelectric_flame_photometer
2. Atomic Spectroscopy,A Guide to Selecting the Appropriate Technique and System www.perkinelmer.com/Atomic Spectroscopy
3. Xiandeng Hou and Bradley T. Jones, Inductively Coupled Plasma/Optical Emission Spectrometry, R.A. Meyers (Ed.) pp. 9468–9485 . A. Walsh (1955).
4.The application of atomic absorption spectra to chemical analysis, Spectrochim. AA. Walsh (1955), The application of atomic absorption spectra to chemical analysis, Spectrochim. Acta 7: 108–117.
5.J.W.Robinson,Recent Advances in Atomic Absorption Spectroscopy, anal.Chem., 1961, 33 (8), pp 1067–1071
6. Bauman, R.P. Absorption Spectroscopy, John Wiley, New York, 1965.
7. Dean, J., Flame Photometry, McGraw-Hill, New York, 1960.
8.Ottaway, J., and A. Ure, Practical Atomic Absorption Spectroscopy, Pergamon, New York, 1983.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









