{ DOWNLOAD AS PDF }
About Authors:
Nehal C. Ghelani*, Ketan Dadhania, Shital Faldu
Department of Quality Assurance,
Smt. R. D. Gardi B. Pharmacy College, Rajkot, Gujarat, India
*nehalghelani10@gmail.com
Abstract
RP-HPLC method was developed for the simultaneous estimation of Olmesartan Medoxmil and Cilnidipine in pharmaceutical dosage form. The separation was achieved Hypersil C18 (250 x 4.6 mm, 5 mm) columnwith Acetonitritle:Phosphate buffer pH 3.6 (70:30 %v/v). Flow rate was maintained at 1.0 ml/ min and UV detection was carried at 270 nm. Retention time for Olmesartan Medoxomil and Cilnidipine was found to be 4.14 min and 7.79 min respectively. The method has been validated for linearity, accuracy and precision. Linearity for Olmesartan Medoxomil and Cilnidipine were in the range of 40 - 200 µg/ml and 10 - 50 µg/ml respectively. The percentage recoveries obtained for Olmesartan Medoxomil and Cilnidipine were found to be in range of 99.96- 100.23 and 98.99-100.03 respectively. The developed method was validated as per ICH guidelines. Developed method was found to be accurate, precise, selective and rapid for simultaneous estimation of Olmesartan Medoxomil and Cilnidipine in pharmaceutical dosage form.
INTRODUCTION
Olmesartan Medoxomil (OLME) (fig. 1a) is the chemically known as, 2,3-dihydroxy-2-butenyl4(1-hydroxy-1-methylethyl)-2-propyl-1-[p-(o-1H-tetrazol-ylphenyl)benzyl]imidazole carboxylate, cyclic 2,3-carbonate. Olmesartan is a prodrug that works by blocking the binding of angiotensin II to the AT1 receptors in vascular muscle; it is therefore independent of angiotensin II synthesis pathways, unlike ACE inhibitors. By blocking the binding rather than the synthesis of angiotensin II, olmesartan inhibits the negative regulatory feedback on renin secretion. As a result of this blockage, olmesartan reduces vasoconstriction and the secretion of aldosterone[1-3]. Cilnidipine(CILNE) (fig. 1b) the chemically known as, o3-(2-methoxyethyl) O5-[(E)-3-phenylprop-2-enyl]2,6-dimethyl-4-(3-nitrophenyl)-1,4dihydropyridine-3,5-dicarboxylate. Cilnidpiine is it reduces the incidense of pedal edema unlike amlodipine. Cilnidipine due to its blocking action at N-type calcium channel dilates both arteriole & venules as a result the pressure in the capillary bed is reduces. The accumulated fluid in the tissues flows back to veins & thus Cilnidipine minimizes the incidence of pedal edema[1-4]. Combination drug products of OLME and CILNI are widely marketed and used in the treatment of hypertension[5-7].Several analytical methods like UV spectrophotometry, HPLC, HPTLC, UPLC have been reported for estimation of OLME & CILNI by single drug and also by combining with other drugs. But there was no any method has been reported till date for the simultaneous estimation of Olmesartan Medoxomil & Cilnidipine using the RP-HPLC method. The present paper describes the development and validation of two analytical methods for simultaneous estimation of Olmesartan Medoxomil & Cilnidipine by RP-HPLC method in tablet dosage form. The proposed method are validated as per the ICH guidelines[10-12]
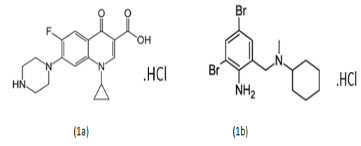
Fig. 1 Chemical structures of the analytes (1a) Olmesartan Medoxomil & (B) Cilnidipine
MATERIALS AND METHODS
Instrumentation
RP-HPLC (LC 100 Cyber lab) having detector LC - UV 100 UV detector . Inertsil C18 (250 x 4.6 mm, 5mm) column was used.WS 100 Workstation software was used.
Material and reagent
Olmesartan Medoxomil (OLME) was obtained from Intas Pharma, Ahmedabad, Gujaratand Cilnidipine (CILNI) bulk powder was kindly gifted by J. B. Chemical, Surat, Gujarat. Acetonitritle HPLC grade was from Rankem Chemical Ltd., Potassium dihydrogen ortho phosphate was from Sulab reagent, Suvidhinath Labs.
Preparation of Combined Standard Stock solution of OLME and CILNI:
40 mg of standard OLME and 10 mg of standard CILNI were weighed and transferred to 100 ml volumetric flask and shake for 10 min in 50 ml of HPLC grade methanol. After that the volume was made upto the mark with methanol to obtain 400 mg/ml of OLME and 100 mg/ml of CILNI. The solution was labeled as 'Stock solution A'
Preparation of combined Working Standard Solution of OLME and CILNI:
From the ‘Stock solution A’ 1 ml of aliquot was pipette out in10 ml volumetric flask and shake to dissolve in methanol. After that the volume was made up to the mark with methanol to obtain 40 mg/ml of Olmesartan Medoxomil and 10 mg/ml of Cilnidipine. The solution was labeled as ' Working stock solution A'.
Preparation of calibration curve:
From the 'Stock solution A' (400 mg/ml of OLME and 100 mg/ml of CILNI ) 1, 2, 3, 4, and 5 ml of aliquot was pipette out in 10 ml volumetric flask and made the volume upto the mark with diluent (mobile phase) to get 40 - 200 mg/ml of OLME and 10 - 50 mg/ml of CILNI. The chromatogram was recorded under the finalized chromatographic conditions as described above after getting a stable baseline. Peak areas were recorded for all the peaks. Calibration curves of OLME and CILNI were constructed by plotting the peak area of OLMEvs. OLME concentration and peak area of CILNIvs. CILNI concentration, respectively.
VALIDATION PARAMETERS
Validation of developed method was carried out as per ICH guideline.[10] Parameters such as Linearity and range, Accuracy, Precision, LOD and LOQ, specificity, system suitability were taken up as tests for analytical method validation.
Linearity and Range:
Linearity of the proposed method was verified by analyzing five combined different concentrations in the range of 40 - 200 mg/ml and 10-50 mg/ml for OLME and CILNI. Each concentration was made five times. The calibration curve of Peak area vs. respective concentration was plotted and regression line equation for OLME and CILNI was calculated.
Precision
The repeatability was evaluated by combined standard solutions of 3 concentration and 3 replicates of each concentration of OLME and CILNI.
The intraday precision of the developed method was evaluated by analyzing combined samples of different concentrations of OLME and CILNI three times on the same day and % RSD was calculated.
The inter day precision was evaluated from the combined concentration of OLME and CILNI on three different days and % RSD was calculated.
Limit of detection (LOD) and limit of quantitation (LOQ)
They were calculated as 3.3 σ/S and 10 σ/S respectively. Where σ is the standard deviation of the response (y-intercept) and S, is the mean of the slope of calibration plot.
Recovery Studies:
The accuracy of the method was performed by conducting the recovery studies (80, 100 and 120%) of pure drugs from marketed formulation, by standard addition method. The actual and measured concentrations were then compared
Specificity:
Specificity is a procedure to detect quantitatively the analyte in the presence of component that may be expected to be present in the sample matrix. Commonly used excipients in tablet preparation were spiked in a pre weighed quantity of drugs and then peak area was measured and calculation was done to determine the quantity of the drugs.
System suitability
Combined standard solutions of OLME ( 120 mg/ml) and CILNI ( 30 mg/ml) were prepared and analyzed six times. Chromatograms were studied for different parameters such as tailing factor, resolution and theoretical plates to see that whether they complies with the recommended limit or out of recommended limit.
Application of Proposed Method to dosage form:
To determine the content of OLME and CILNI in commercial tablets (each tablet containing 40 mg OLME and 10 mg CILNI), 20 tablets were weighed and finely powdered. A quantity of powder equivalent to 40 mg of OLME and 10 mg of CILNI was weighed accurately and transferred to 100 ml volumetric flask and the volume was made up with the methanol and then filtered through 0.5 mm whatman paper. From the above prepared solution, further dilutions were prepared in the linearity range using diluent(mobile phase). The chromatogram was taken at selected wavelengths and peak areas were found out. The analysis was done in five replica.
RESULTS AND DISCUSSION
Method Validation:
The linearity range for OLME and CILNI were 40 - 20 μg/mL and 10 - 50 μg/mL respectively. The results of the recovery studies are found to be satisfactory for OLME and CILNI and shown in Table 1 and 2 respectively. The result of assay procedure obtained was showed in Table 3. Summary of Other validation parameters including Repeatability, Intraday, Interday, LOD and LOQ, system suitability were found to be satisfactory and are shown in Table 5. Specificity is proven by comparing the chromatogram of Diluent, standard solution and test preparation solution to show that there was no any interference of excipients with the peak of OLME and CILNI, as shown in figure 4, 5, 6, and 7.
CONCLUSION
RP-HPLC method was developed and validated for the determination of OLME and CILNI in their combined pharmaceutical dosage form. The present RP-HPLC method can be considered simple and rapid to apply. This method was validated as per ICH guideline and fulfill the criteria of validation.
ACKNOWLEDGEMENT
The authors are thankful to Smt. R. D. Gardi B. Pharmacy College, Rajkot, Gujarat, India for providing necessary facilities to carry out this work. We are also thankful to Intas Pharma, Ahmedabad &J. B. Chemaicals, Surat. for providing the free gift samples of OLME and CILNI which was required for our research work..
FIGURE AND TABLES
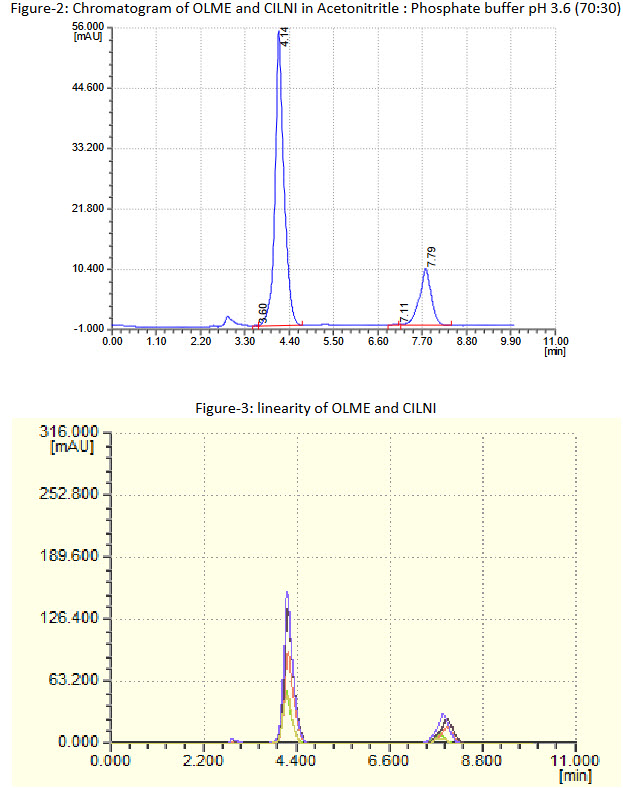
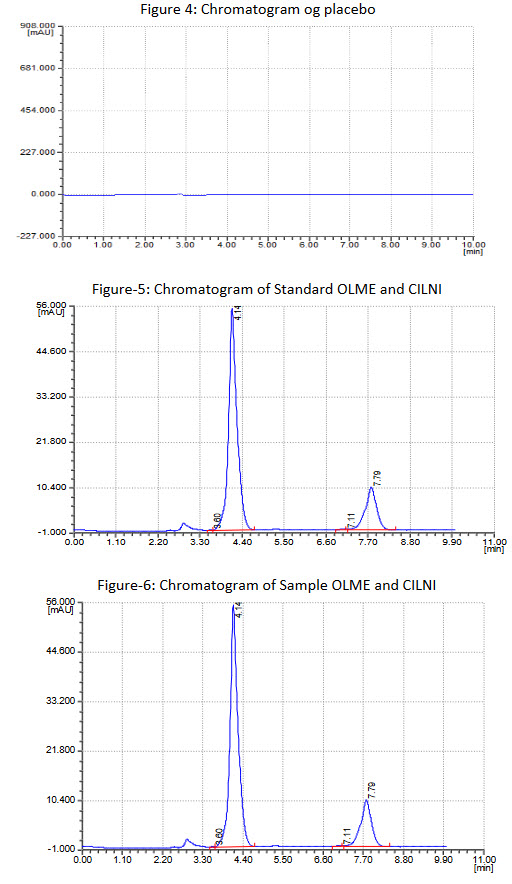
Table-1 Result of Recovery Studies for OLME in dosage form:
|
Amount of OLME in mixture (μg/ml) |
Amount of Std OLME added (μg/ml) |
Total amount of OLME (μg/ml) |
Total amount of OLME found (μg/ml) Mean* ± SD |
%Recovery |
|
120 |
96 |
216 |
216.3 ± 0.213 |
100.1 |
|
120 |
120 |
240 |
239.9 ± 0.152 |
99.96 |
|
120 |
144 |
264 |
264.3 ± 0.416 |
100.23 |
[*=mean value of 3 determination]
Table-2 Result of Recovery Studies for CILNI in dosage form:
|
Amount of CILNI in Mixture (μg/ml) |
Amount of Std CILNI added (μg/ml) |
Total amount of CILNI (μg/ml) |
Total amount of CILNI found (μg/ml) Mean* ± SD |
%Recovery |
|
30 |
24 |
54 |
54.04 ± 0.026 |
100.07 |
|
30 |
30 |
60 |
59.4 ± 0.545 |
98.99 |
|
30 |
36 |
66 |
65.69 ± 0.594 |
99.53 |
[*=mean value of 3 determination]
Table-3: Analysis of OLME and CILNI in dosage form:
|
Tablet dosage form |
Label claim(mg) |
%Recovery ± SD (% of label claim*) |
||
|
OLME |
CILNI |
OLME |
CILNI |
|
|
|
40 mg |
10 mg |
100.12% |
98.98% |
[*=mean value of 5 determination]
Table-4: Regression Characteristics:
|
Characteristics |
OLME |
CILNI |
|
Linearity (μg/ml) |
40 - 200 |
10 - 50 |
|
Regression Equation |
y = 1474.x + 29516 |
y = 1686.x + 6113 |
|
Slope |
1474 |
1686 |
|
r2 |
0.997 |
0.998 |
|
Intercept |
29516 |
6113 |
|
S.D. of Intercept |
58.3209 |
60.0125 |
TABLE-5: VALIDATION PARAMETERS:
|
Parameters |
OLME |
CILNI |
|
Repeatability(%RSD) (n=6) |
1.984 |
0.808 |
|
Precision (%RSD) |
|
|
|
Intra-day (n=3) |
0.236 – 1.612 |
0.085 – 0.876 |
|
Inter-day (n=3) |
0.102 – 0.287 |
0.065 – 0.430 |
|
LOD (μg/ml) |
0.1305 |
0.1174 |
|
LOQ (μg/ml) |
0.3956 |
0.3559 |
|
% Recovery (n=3) |
99.96%-100.23% |
98.99%-100.07% |
|
Assay (mean ± S.D.) Theoritical plates Tailing factor Resolution Retention time |
100.12 ± 0.9947
1433.09 1.09 1.66 4.14 |
98.98 ± 1.0530
2819.50 0.80 1.54 7.79 |
LOD: Limit of Detection, LOQ: Limit of Quantitation, R.S.D.: Relative standard deviation, S.D.: Standard deviation
REFERENCES:
(1) Tripathi, KD, “Essential of Medical Pharmacology”, 4th edition, new Delhi, Jaypee brothers, 2001, pp.521, 540
(2) The Merck Index – An Encyclopedia of Chemicals, Drugs and Biologicals, 13th Edn. Merck &Co., INC, 2001, pp 1223, 395
(3) En.wikipedia.org/Wiki/Olmesartan_medoxomil
(4) En.Wikipedia.org/Wiki/Cilnidipine
(5) drugaupdate.com/.../Olmesaetan%20 medoxomil
(6) Thedu.in/…/62697-olmark-40-mg-olmesartan-medoxomil
(7) drugaupdate.com/generic/view/1129
(8) European director for the quality of medicines, Pharmaeuropia 10(4), 547(1988), acerred online at pheur.org/. European pharmacopoeia (2006)
(9) Rang HP, Dale MM, and Ritter JM, Pharmacology, 6th Edn, Churchill Livingstone, New York, 2007, pp 294 – 315.
(10) Chatwal GR, Anands, Ultraviolet- Spectroscopy, Instrumental Methods of Chemical analysis, Mumbai, Himalaya Publishing House, 2000, pp 180 – 198.
(11) Sharma S, Sher P, Badve S, Atmaram PP. Recent advances in Spectroscopic technique, 6th Edn, AAPS Pharm Sci Tech 2005, E 618-E 625.
(12) Backett AH, and Stenlake JB, Practical Pharmaceutical Chemistry, First Edition, Reprint, 2004, CBS Publishers and Distributors, New Delhi, 2
REFERENCE ID: PHARMATUTOR-ART-2196
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 7 Received On: 17/04/2014; Accepted On: 01/05/2014; Published On: 01/07/2014How to cite this article: NC Ghelani, K Dadhania, S Faldu; Analytical Method Development and Validation for Simultaneous Estimation of Olmesartan Medoxomil and Cilnidipine in their Combined Pharmaceutical Dosage form by RP-HPLC Method; PharmaTutor; 2014; 2(7); 142-148 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









