{ DOWNLOAD AS PDF }
ABOUT AUTHORS
K.GOPALASATHEESKUMAR1*, S.KOMALA2 AND M.MAHALAKSHMI2
1KMCH college of Pharmacy, Kovai Estate, Kalapatti Road, Coimbatore-641048, Tamil Nadu, India.
2Jaya College of Paramedical sciences, College of Pharmacy, Thiruninravur, Chennai – 602024, Tamil Nadu, India.
*gskpungai@gmail.com
ABSTRACT
The review was carried out to discuss in detail about the polymeric nanoparticles for diabetic treatment. The diabetes is the chronic metabolic disorder characterized by the deficiency of insulin production. The various treatments are available for the diabetes and the nanoparticles are having the several advantages. The various types of nanoparticles are available for the anti-diabetic drugs; the polymeric nanoparticles are the one of the most commonly used nanoparticles. The polymeric nanoparticles are commonly 10-1000nm in size. The polymeric nanoparticles are formulated by drug with the polymers. The main advantages of the polymeric nanoparticles are the simplest preparation method, targeted delivery, the minimizing of the dose and high therapeutic efficiency. In this review was mainly can be focused on advantages, disadvantages of polymeric nanoparticles, various polymers, various formulation techniques, diabetes disease profile, insulin production, various anti-diabetic drugs and the polymeric nanoparticle formulation of anti-diabetic drugs.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2549
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 12 Received On: 13/08/2017; Accepted On: 13/09/2017; Published On: 01/12/2017 How to cite this article: Gopalasatheeskumar K, Komala S, Mahalakshmi M;An Overview on Polymeric Nanoparticles used in the treatment of Diabetes Mellitus; PharmaTutor; 2017; 5(12);40-46 |
INTRODUCTION
Nanomedicine is a subdivision of nanotechnology, which uses small particles that are more than 10 million times smaller than the human body. In nano-medicine, these particles are greatlylesser than the living cell. Because of this, nanomedicine presents many innovativechances in the fight against all types of cancer, neurodegenerative disorders and other diseases. (SovanLal et al., 2011)
Liposomes are concentric bilayered vesicles in which an aqueous volume is completelysurrounded by a membranous lipid bilayer mainly composed of natural or synthetic phospholipids. Nanocrystals are aggregates of about hundreds or thousands of particles that combine in a crystal-like form, composed of pure drug with only a thin coating comprised of surfactant or combination of surfactants.(Nishikant et al., 2012, Senthilnathan et al., 2016) Solid Lipid Nanoparticles contain a solid lipid medium, where the drug is normally incorporated, with an average diameter below 1 μm. (Nagarajan et al., 2015) Dendrimers, anexceptional class of polymers, are extremely branched macromolecules whose size andshape can be exactlymeasured. Metallic nanoparticles are the having the metallic compounds like gold, silver, selenium, iron etc. the green synthesized nanoparticles are having the plant source of nanoparticles. (Nagavarma et al., 2012, Mohanraj et al., 2006)
Polymeric nanoparticles are commonly 10-1000nm in dimension. These polymeric nanoparticles are formulated from the polymers, which have the nature of bio-adaptability,bio-comptabilityand bio-degradable. The drug is dissolved, entrapped, encapsulated to a nanoparticle medium. The nanoparticles, nano-spheres or nano-capsules are obtained by depending up on the preparation. In nano-capsule system, the drug is limited to a cavity enclosed by evenpolymer layer, while the nano-shell contains of medium, in which the drug is physically and uniformly dispersed.(Konwar et al., 2013, Neha et al., 2013)
Advantages of polymeric nanoparticles
- Preparation method is easy
- Targeted drug delivery method
- Because of their lesser size Nanoparticles enter small capillary and are taken up through the cell which allows for well-organized drug buildup at the target sites in the body. (Nishikant et al., 2012)
- Good control of over size and size distribution.
- Good protection of the compressed drug.
- Retaining of the drug at active site.
- clearance time is longer
- High therapeutic efficacy.
- High bioavailability.
- Dose proportionality.
- Faster dissolution of active agents
- Faster dissolution generally equates with greater bioavailability.
- Lesser drug doses.
- Less toxicity.
Disadvantages of polymeric nanoparticles
- Wide use of polyvinyl alcohol as a detergent –subjects with toxicity.
- Limited targeting capabilities.
- Termination of therapy is not possible.
- Cytotoxicity. (Naik et al., 2012)
- Pulmonary inflammation and pulmonary carcinogenicity.
- The trouble of autonomic inequity by nanoparticles consumingstraightresult on heart and vascular function.
Therapeutic Applications of Nanoparticles
* Carriers of drugs and biological agents
* Carriers of gene and DNA
* Carriers of antigens & vaccines
* Controlled and targeted drug delivery
* Carriers of diagnostic agent
Polymers Used for Preparation of Polymeric Nanoparticles
The picture is having four types of polymers are can be used for the polymeric nanoparticles formulations. The natural types of polymers are the obtained from the natural sources like animal or plants. The synthetic polymers are the prepared by the chemical synthesis methods. (Senthilnathan et al., 2015)
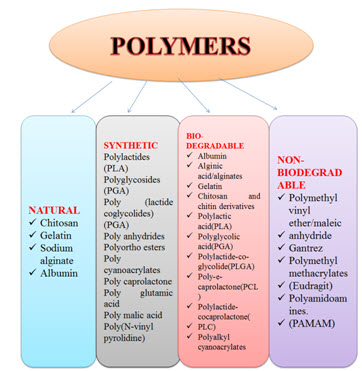
Figure 1: Types of polymers used for polymeric nanoparticles
Preparation methods for polymeric nanoparticles
The following preparation methods are the commonly used for the polymeric nanoparticles.
Emulsion-Solvent Evaporation Method:
This methodinvolves two steps. The first step is emulsification of the polymer solution into an aqueous phase. In second step polymer solvent is evaporated, making polymer precipitation as nanospheres. The nano particles are collected by ultracentrifugation and washed with distilled water to remove additivedeposit or any free drug and lyophilized for storage.9Modification of this method is high pressure emulsification-solvent evaporation method. This method involves preparation of an emulsion which is then subjected to homogenization under high pressure followed by complete stirring to eliminate organic solvent.The size of nanoparticles is controlled by regulating the stirring rate, type and amount of dispersing agent, viscosity of organic phase and aqueous phase and temperature.
Double Emulsion and Evaporation Method:
The disadvantage of emulsion-solvent evaporation method is poor entrapment of hydrophilic drugs. The double emulsion technique is overcomes this disadvantage and encapsulate the hydrophilic drugs, which is done by the addition of aqueous drug solutions to organic polymer solution under vigorous stirring to form w/o emulsions. This w/o emulsion is added into second aqueous phase with continuous stirring to form the multiple emulsions (w/o/w emulsion). The emulsion then allowed to removal of solvent by evaporation,nanoparticles are isolated by centrifugation at high speed. The formed nanoparticles are carefully washed before lyophilisation.
Emulsions- Diffusion Method:
The polymer is dissolved in a moderately water-miscible solvent (for example propylene carbonate, benzyl alcohol), and saturated with water to ensure the initial thermo-dynamic equilibrium of both liquids.(Anusha et al., 2011) Then, the polymer-water saturated solvent phase is emulsified in aqueous solution having stabilizer, leading to solvent diffusion to the external phase and the developmentof nanoparticles, according to the oil-to-polymer ratio. Finally, the solvent is removed by evaporation or filtration, as said by its boiling point.
Salting Out Method:
In this method polymer and drug are primarilydissolved in a solvent which is afterward emulsified into an aqueous gel containing the saltingout agent (electrolytes,for example magnesium chloride and calcium chloride, or non- electrolytes as sucrose) and a colloidal stabilizer for example polyvinylpyrrolidone or hydroxyethylcellulose. This o/w emulsion is dilute with anadequate amount of water or aqueous solution to increase the dispersion of solvent into the aqueous phase, hence the formation of nanoparticles.
Solvent Displacement / Precipitation method:
Solvent displacement includes the precipitation of a preformed polymer from an organic solution and the diffusion of the organic solvent in aqueous medium in the presence or absence of the surfactant. Polymers, drug, and lipophilic surfactantsare dissolved in a semi-polar water miscible solvent for example acetone or ethanol. The solution is now poured or injected into an aqueous solution having stabilizer above magnetic stirring. Nano particles are formed immediatelyby the rapid solvent diffusion. The solvent is then removed from the suspensions under reduced pressure. This method is well suited for most of the poorly soluble drugs.
Dialysis
This method is created on a solvent displacement mechanism which includesextra toolsfor example dialysis tubes or semi-permeable membranes through suitable molecular weight cut-offwhich helpby way of a physical barrier for the polymer. Therefore, dialysis is achieved against anon-solvent of the polymer miscible with the polymer solvent. The movement of thepolymer solvent through the membrane induces a progressive loss of solubility of thepolymer leading to the development of homogeneous nano suspensions.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Supercritical Fluid Technology
The supercritical fluid technology is globallyharmless method for the making of polymeric nanoparticles. The usefulness of supercritical fluid has more ecologicalapproachablesolvents, and has the possible to produce polymeric nanoparticles with high purity andwithout any trace of organic solvent. This method is done by following two methods
- Rapid expansion of supercritical solution.
- Rapid expansion of supercritical solution into liquid solvent.
Rapid Expansion of Supercritical Solution (RESS)
In this method, the solute is liquefied in a supercritical fluid to form solution, and then followed by the rapid development of the solution crossways the capillary nozzle into the ambientair. The rapid pressure reduction in the expansion results the homogeneous nucleation and the formation of fine dispersed particle.
Rapid expansion of supercritical solution in to liquid solvent
This method is nearly like to the above method but in different to the RESS, the expansion of the supercritical solution in to a liquid solvent in its place of ambient air. The primary nano-sized particles are not allowed to produce in the expansion jet due to the occurrenceof the liquid solvent. For example, poly(heptadecafluorodecylacrylate particles were produced using water as the solvent in which were expanded the supercritical solution and precipitated the polymer. It was presented that the particle development results from the aggregation of originally formed nanoparticles.
Ionic gelation technique/coacervation of hydrophillic polymers
The decomposable hydrophilic polymers for example chitosan, gelatin and sodium alginate are used to formulate polymeric nanoparticles through ionic gelation method. This method containscombination of double aqueous phases, one is thepolymer chitosan and the other is a poly anion sodium tri-polyposphate. The tool of this method is, positive charge amino group of chitosan interacts with the negative charge tripolyposphate to form coacervate with in its size range of nanometer. The coacervation of polymer and particles are made by the electrostatic interfaceamongthe two aqueous phases. In ionic gelation technique the material undergoing alteration from liquid to gel because of the ionic interaction state at room temperature.
DIABETES MELITUS
Diabetes mellitus has been known to manhood for over 2000 years. It is probable to become one of the most predominant and economicallysignificant diseases of the 21stcentury in together the advanced and emerging nations. Diabetes Mellitusis a group of metabolic disorder considered by a wholelack of insulin, a comparative lack of insulin, or insulin resistance, which then results in hyperglycemia. (Deopa et al., 2013)
Diabetes mellitus is produced by changed metabolism of carbohydrate, lipid and lipoprotein subsequent from the fault in insulin secretion and action; it is characterized through symptom likehyperglycemia, glycosuria, polyphagia, polyurea, polydipsia, gradual loss of weight, fatigue, cramps, blurred vision, constipation, and candidiasis are prominent.(Harris et al., 1998) It is the most predominant chronic disease in the world affecting nearly 100 million people of the population where 5-10% having type 1 while 90-95% of them suffers from type 2 diabetes mellitus.Diabetes leads to many health complications such as hyperlipidemia, hypertension and atherosclerosis.
Classification of Diabetes
There are mainly four types of diabetes mellitus
• Type I diabetes or insulin dependent diabetes mellitus
• Type II diabetes or non-insulin dependent diabetes mellitus
• Gestational diabetes
• Genetically modified diabetes.
Type I diabetes can occur in any age. It is an immune mediated disease subsequent from destruction in β-cells of pancreas which leads to insufficientendogenous insulin making. Type II diabetes or non-insulin dependent diabetes mellitus is the most common type affecting old and obese individualproduced either by insulin resistance or lacking insulin secretion. It is characterized by hyperglycemia in the presence of hyperinsulinemia due to peripheral insulin resistance.A third type of diabetes gestation diabetes mellitus is first recognize during pregnancy where hyperglycemic disorder developsin women who doesn’t have diabetes result from an insufficient insulin supply to meet tissue demand for normal blood glucose regulation.(Fowler et al., 2008) Fourth type of diabetes is genetically modified diabetes mellitus there occur defects in β cell function or mutation of insulin receptor and may lead to diabetes. Other rare types of diabetes comprisepersons caused by surgery, drug used (e.g. antihypertensive vasodilator diazoxide, lower dose of thiazides, corticosteroids in high doses, high dose of anabolic androgens, oral contraceptives, streptozotocin, theophylline, asprin, isoniazid, alloxan, nalidixic acid ), malnutrition, infection and other illness
Insulin and its secretion
The insulin is a polypeptide hormone its molecular weight of 6000 Da. It is initially produced as a single molecule (pre-proinsulin) contains 110 amino acids released by the pancreatic β-cells. These are regulates the glucose levelin the system. It contains two polypeptide chains A and B. Chain A contains 21 amino acids and chain B having 30 amino acids. Two disulfide bonds covalently bind the chain where chain A containsan internal disulfide bridge. Hormone insulin is produced as pre- proinsulin in rough endoplasmic reticulum further by proteolysis it change to pro-insulin and then to insulin. After pre-proinsulin has passed through the endoplasmic reticulum, 24 amino acids are detached by enzymatic action from one end of chain, leaving anotherform pro-insulin that undergoesfolds and bonds to passes towardsGolgi body where the central section of 33 amino acids is detached by the action of the enzymespro-hormone convertase1 and 2 converting to final structure of insulin. (Sanjukta et al., 2015)
Hypoglycemic Drugs
Anti-diabetic drug acts by two main mechanisms one is stimulation of β-cells in pancreatic islet to release insulin and another one is increase the sensitivity or amount of insulin receptor.(Fowler et al., 2008) Preoperatively, oral hypoglycemic agents, especially those that motivate insulin secretion, such as sulfonylurea and meglitinide agents, have possible for creating hypoglycemia during fasting prior to surgery. The sulfonylurea agents (e.g. glipizide, glyburide, glimepiride) are generallygiven oral hypoglycemic agents for the action of type 2 diabetes. These agents act by bind to the ATP-dependent potassium (KATP) channel in the pancreatic s-cells, leading to end of these channels and motivating insulin release. Thus, pancreatic β-cells are progressively responsive to glucose absorptions and insulin release is increased. Metformin, a biguanide oral hypoglycemic agent, is generally used for treatment of type II diabetes mellitus.α-glucosidase inhibitors are additional class of drugs that comprises compound like acarbose which delay the intraluminal production of glucose. Acarbose competitively stops α-glucosidase that is connected with the brush border membrane of the small intestine and responsible for the digestion of complex polysaccharides and sucrose. Natural compounds may be anothermanagement as they can be comprised in everydayfood and can be taken in larger amount without any risk. Many plants areidentified in traditional medicine of different culture to be used for their anti-diabetic property.
Polymeric Nanoparticles for diabetic treatment
The polymeric nanoparticles are the advanced method of the treatment of diabetes mellitus which having the several advantages and the novel drug delivery system. The using of the polymeric nanoparticles is the effective treatment of diabetes mellitus. The table.1 is having the various polymeric anti-diabetic drugs, polymers used for the formulation and the formulation methods.
Table 1: Polymeric Nanoparticles for diabetic treatment
|
s.no |
Drug used |
Polymer used |
Method of preparation |
Reference |
|
1 |
Metformin |
Ethylcellulose (EC), Poly (lactic-co-glycolic acid) (PLGA), Poly (methyl methacrylate) (PMMA), and Chitosan |
solvent evaporation method |
DhanalekshmiUnnikrishnanet al., 2015 |
|
2 |
GLIPIZIDE |
Polycaprolactone |
Emulsification-solvent evaporation technique |
JitendraNaik., et al. 2013 |
|
3 |
Glipizide |
Eudragit RL100 |
solvent evaporation |
Priyanka Saharan et al., 2015 |
|
4 |
Pioglitazone Hydrochloride |
Chitosan |
Solvent Displacement Method |
A. Umar faruksha et al., 2013. |
|
5 |
Glipizide |
PLGA and Eudragit RS 100 |
Single emulsion solvent evaporation method |
Pratap Naha et al., 2012. |
|
6 |
Glibenclamide |
Poly (lactic-co-glycolic) acid |
emulsification solvent evaporation method. |
AmulyaratnaBehera et al., 2012. |
|
7 |
Insulin |
Chitosan |
Ionotropic gelation method |
Mounica Reddy M et al., 2012 |
|
8 |
Human insulin (Mw ~ 5800 Da) |
PLGA |
double emulsion method |
YaseminBudama-Kilinc et al., 2017 |
|
9 |
Human insulin100 IU/mL) |
polycaprolactonetriol |
precipitation polymerization method |
Pijush Kumar Paul et al., 2017 |
|
10 |
Porcine insulin (30 IU/mg) |
Chitosan |
polyelectrolyte ionotropic gelation |
ZhiyangK et al., 2015 |
|
11 |
Costusspeciosus leaves |
polylactic- co-glycolic acid (PLGA) |
solvent displacement technique |
Wagdy K. B. Khalil et al., 2014 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
The polymeric nanoparticles are formulated by drug with the polymers. The main advantages of the polymeric nanoparticles are the simplest preparation method, targeted delivery, the minimizing of the dose and high therapeutic efficiency. In this review was concluded that the polymeric nanoparticles are the advanced method of the treatment of diabetes mellitus.
REFERENCES
1) Amolkumar Lokhande, Satyendra Mishra, Ravindra Kulkarni and Jitendra Naik (2013); Formulation and Evaluation of Glipizide Loaded Nanoparticles; Int J Pharm Pharm Sci; Vol. 5 No. 4; 147-151.
2) Anusha R., Somasundaram I., Ravichandiran V., Kausalya J., Joseprakash D and Senthilnathan B. (2011); Effect of Cholesterol and Surfactant Concentration on Solid Lipid Nanosuspension of Ropinirole Hydrochloride; Journal of Pharmacy Research; Vol. 4 No. 6; 1606-1609.
3) Deopa Deepika, Sharma Kumar Satish and Singh Lalit. (2013); Current updates on Anti-Diabetic Therapy; JDDT; Vol. 3 No. 6; 121-126.
4) Ebtiha F. Alamoudi, Wagdy K.B Khalil, Inas S. Ghaly, Nagwa H.A Hassan and Ekram S. Ahmed. (2014); Nanoparticles from of Costus speciosus extract Improves the Antidiabetic and AntilipidemicEffects Against STZ-induced Diabetes Mellitus in Albino Rats; Int. J. Pharm. Sci. Rev. Res.; Vol. 29 No. 1; 279-288.
5) Fowler M. J. (2008); Microvascular and macrovascular complication of diabetes; Clinical diabetes; Vol. 26 No. 2; 77-82.
6) Harris M.I., Flegal K.M., Cowie C.C., Eberhardt M.S., Goldstein D.E., Little R.R., Wiedmeyer H.M. and Byrd Holt D.D.(1998); Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the Third National Health and Nutrition Examination Survey 1988–1994; Diabetes Care; Vol. 21; 518–524.
7) Konwar Ranjit and Ahmed Abdul Baquee.(2013); Nanoparticle: A review of preparation, characterization and application; IRJP; Vol. 4 No.4;48-52.
8) Lokhande A.K., Sathyendra and Kulkarni R.(2013); Formulation and evaluation of Glipizide loaded nanoparticles; Int J Pharm Pharm Sci; Vol. 5 No.4; 147-151.
9) Mohanraj V.J. and Chen Y.(2006); Nanoparticles – a review; Tropical journal of pharmaceutical research; Vol. 5 No.1; 561-573.
10) Mounica Reddy M., Shanmugam V. and Rajesh Kaza.(2012); Design and characterization of Insulin Nanoparticles for oral delivery; IJIPSR; Vol. 3 No. 3; 238-243.
11) Nagarajan E., Shanmugasundaram P., Ravichandiran V., Vijayalakshmi A., Senthilnathan B. and Masilamani K.(2015); Development and Evaluation of Chitosan Based Polymeric Nanoparticles of an Antiulcer Drug Lansoprazole; JAPS; Vol. 5 No. 4; 20-25.
12) Nagavarma B.V.N., Hemant K.S. Yadav, Ayaz A., Vasudha S. and Shivakumar H.G.(2012); Different techniques for preparation of polymeric nanoparticles- A review; Asian J Pharm Clin Res.; Vol. 5 No.3; 16-23.
13) Neha Yadav, Sunil Khatak and Udai Vir Singh S.A.(2013); Solid lipid nanoparticles- A review; Int J App Pharm; Vol. 5 No.2; 8-18.
14) Naik J.B. and Mokale V.J.(2012); Formulation and evaluation of Repaglimide nanoparticles as sustained release carriers; International Journal of Pharmaceutical Sciences; Vol. 1 No. 5; 259-266.
15) Nishikant C. Shinde, Nisha J. Keskar and Prashant D. Argade.(2012); Nanoparticles: Advances in Drug Delivery systems; RJPBCS; Vol. 3 No. 1; 922-929.
16) Pijush Kumar Paul, AlongkotTreetong and Roongnapa Suedee.(2017); Biomimetic insulin-imprinted polymer nanoparticles as a potential oral drug delivery system; Acta Pharm; Vol. 67; 149–168.
17) Pratap C. Naha, Hugh J. Byrne and Amulya K. Panda.(2012); Role of polymeric excipients on controlled release profile of Glipizide from PLGA and Eudragit RS 100 Nanoparticles; JND; Vol. 1; 1–9.
18) Priyanka Saharan, Bhatt D.C., Saharan S.P. and Kavita Bahmani.(2015); Preparation and characterization of antidiabetic drug loaded polymeric nanoparticles; Der Pharma Chemical; Vol. 7 No. 12; 398-404.
19) Sanjukta Duarah, Kunal Pujari, Jyotirmoy Ghosh, Dhanalekshmi and Unnikrishnan.(2015); Formulation and Evaluation of Metformin Engineered Polymeric Nanoparticles for Biomedical Purpose; RJPBCS;Vol. 6 No. 3; 1005-1019.
20) Senthilnathan B., Ameerkhan H, Aswini Sofia P.I., Abirami M., Bharath T., Ajithkumar T. and Maheswaran A.(2015); Review on various approaches on Preparation, Characterization and Applications of Polymeric Nanoparticles; WJPR; Vol. 4 No.6; 645-663.
21) Senthilnathan B., Maheswaran A., Gopalasatheeskumar K., Masilamani K. and Raihana Z. Edros.(2016); Formulation and Evaluation of Pregabalin Loaded Eudragit S100 Nanoparticles; IJETS; Vol. 6 No.1; 64-70.
22) Sovan Lal Pal, Utpal Jana, Manna P.K., Mohanta G.P. and Manavalan R.(2011); Nanoparticle: An overview of preparation and characterization; JAPS; Vol. 1 No.06; 228-234.
23) Umar Faruksha A. and Vetrichelvan T.(2013); Formulation, Characterization and Optimization of Pioglitazone Hydrochloride Nanoparticles by Solvent Displacement Method Using 32 Factorial design; Int J PharmTech Res; Vol. 5 No. 2; 754-766.
24) Yasemin Budama Kilinc, Rabia Cakir-Koc and Ozlem Horzum Bayir.(2017); The Cytotoxicity, Characteristics, and optimization of Insulin-loaded Nanoparticles; Orbital: Electron. J. Chem.; Vol. 9 No.1; 66-71.
25) Zhiyang Ke, Han Guo, Xi Zhu, Yun Jin and Yuan Huang.(2015); Efficient Peroral Delivery of Insulin via Vitamin B12 Modified Trimethyl Chitosan Nanoparticles; JPPS; Vol. 18 No. 2; 155 – 170.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









