 About Authors:
About Authors:
Alpeshkumar J.Shiroya*
Bhagwan Mahavir College Of Biotechnology,
Surat
alpeshshiroya45@yahoo.in
ABSTRACT
Laccases are an interesting group of multi-nuclear copper-containing oxidoreductases, which have been subject of intensive research in last decades due to their ability to oxidize both phenolic and nonphenolic lignin related compounds as well as highly recalcitrant environmental pollutants, which makes them very useful for their application to several biotechnological processes. These oxidase enzymes catalyze oxidation of substrates at the expense of molecular oxygen and produce water as the only by-product. Lacasses are present in higher plants, bacteria, fungi, insects and lichens. The oxidative ability of laccases is employed in a number of industrial and environmental applications including bioremediation, food technology, nanobiotechnology, medicine, pulp and paper industry, textile industry, and cosmetology. Owing to its versatility, laccase is continuously under investigation for new applications. In the recent years, laccases are also used as cleaning agents for certain water purification systems, as catalysts for the manufacture of anti-cancer drugs and even as ingredients in cosmetics. More recently, it is also used in the design of biosensors, biofuel cells, as a medical diagnostics tool and bioremediation agent to clean up herbicides, pesticides and certain explosives in soil. This paper reviews the occurrence, mode of action, general properties, production, immobilization, molecular cloning, and important application of laccases within different industrial and biotechnological area.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1419
INTRODUCTION
In the recent years, the use of enzymes in the diverse fields of industrial application has been increased. Many of such potential enzymes are widely distributed in the nature; laccases are one among them. Laccases are the oldest and most studied enzymatic systems [1]. Laccase is the most widely distributed of all the large blue copper-containing proteins. These enzymes contain 15–30% carbohydrate and have a molecule mass of 60–90 kDa with acidic isoelectric point around pH 4.0 [2]. This feature may contribute to the high stability of the enzyme [3].
Although oxidation reactions are essential in several industries, most of the conventional oxidation technologies have the following drawbacks: non-specific or undesirable side reactions and use of environmentally hazardous chemicals. This has impelled the search for new oxidation technologies based on biological systems such as enzymatic oxidation. These systems show the following advantages over chemical oxidation: enzymes are specific and biodegradable catalysts and enzyme reactions are carried out in mild conditions. Enzymatic oxidation techniques have potential within a great variety of industrial fields including the pulp and paper, textile and food industries. Enzymes recycling on molecular oxygen as an electron acceptor are the most interesting ones [6]. Thus, laccase is a particularly promising enzyme for the above-mentioned purposes.
Laccases (p-diphenol : dioxygen oxidoreductases; benzenediol dioxygen oxidoreductases; EC 1.10.3.2) are member of large blue copper proteins or the blue copper oxidases; other enzymes in this group are the plant ascorbate oxidase and the mammalian plasma protein ceruloplasmin [4,5] Laccases are defined in the Enzyme Commission (EC) nomenclature as oxidoreductases which oxidize diphenols and related substances and use molecular oxygen as an electron acceptor. Laccases catalyze the oxidation of a variety of phenolic compounds, as well as diamines and aromatic amines, with concomitant reduction of molecular oxygen to water [4]. The syringaldazine [4-hydroxy-3,5-dimethoxy benzaldehyde azine] is considered the substrate oxidized only by laccase enzyme [4]. Thus, several organic compounds which contain hydroxil, acid, or amino groups can act like substrates [7]. Recently a novel polyphenol oxidase with laccase like activity was mined from a metagenome expression library from bovine rumen microflora [8].
[adsense:468x15:2204050025]
Concerning their use in the biotechnology area, Laccases play an important role in food industry, paper and pulp industry, textile industry, synthetic chemistry, cosmetics, soil bioremediation and biodegradation of environmental phenolic pollutant and removal of endocrine disruptors [9]. These enzymes are used for pulp delignification, pesticide or insecticide degradation, organic synthesis [10], waste detoxification, textile dye transformation, food technological uses, and biosensor and analytical applications.In addition, laccases can be used in a great variety of process, mainly related to the bioremediation such as pulp delignification [11, 12, 13], decolourization and detoxification of textile dyes [14, 15, 16, 17], bioremediation of xenobiotic compounds [18, 19, 20], treatment of wastewater [21, 22] and treatment of other pollutants such as polycyclic aromatic hydrocarbons (PAHs) [23, 24, 25, 26]. This enzyme is very specific, ecologically sustainable and a proficient catalyst.
The biotechnological use of laccase has been expanded by the introduction of laccase-mediator systems, which are able to oxidise non-phenolic compounds that are otherwise hardly or not oxidised by the enzyme alone.Laccase production can be considerably stimulated by the presence of inducers (mainly aromatic or phenolic compounds related to lignin or lignin derivatives) such as veratryl alcohol, 2,5-Xylidine, guaiacol, gallic acid and ferulic acid, ethanol and copper. Being energy-saving and biodegradable, laccase-based biocatalysts fit well with the development of highly efficient, sustainable, and eco-friendly industries.
Recently laccases have been efficiently applied to nanobiotechnology due to their ability to catalyze electron transfer reactions without additional cofactor. The technique for the immobilization of biomolecule such as layer-by-layer, micropatterning, and self-assembled monolayer technique can be used for preserving the enzymatic activity of laccases. The main aim of this review is to summarizethe potential industrial, environmentaland biotechnological applications of laccase enzyme.
SOURCES OF LACCASES AND THEIR PHYSIOLOGICAL ROLES
Laccases are ubiquitous enzymes present in higher plants, bacteria, fungi, insects and lichens [27, 28].
Plants
Yoshida first described laccase activity in 1883 in the sap of the Japanese lacquer tree, Rhus vernicifera [4, 29]. In plants, laccases have been identified in trees, cabbages, turnips, beets, apples, asparagus, potatoes, pears, and various other vegetables [29]. Laccases have subsequently been discovered from numerous other plants, for example, sycamore maple (Acer pseudoplatanus)[30], poplar(Populus euramericana)[31], tobacco [32], peach [33] and loblolly pine (Pinus taeda) [34].Cell cultures of Acer pseudoplatanus have been shown to contain eight laccases, all expressed predominantly in xylem tissue [35]. Other reports are on the presence of a laccase in leaves of Aesculus parviflora [36]and in green shoots of tea [37]. Other higher plant species also appear to contain laccases, although their characterization is less convincing [38].
Plant laccases are found in the xylem tissue, where they presumably oxidize monolignols in the early stages of lignifications [34, 39]. Besides lignification, plant laccases have been shown to be involved in the first steps of healing in wounded leaves [32] and in the mechanism of defense against external conditions [40].
Fungi
In 1896 laccase was demonstrated to be present in fungi for the first time by both Bertrand and Laborde [4, 29]. Laccases have been isolated from Ascomyceteous, Deuteromycteous and Basidiomycetous fungi to which more than 60 fungal strains belong [13]. In fungi, laccases appear more than the higher plants. Laccase from Monocillium indicum was the first laccase to be characterized from an Ascomycete showing peroxidative activity [42]. The white-rot Basidiomycetes fungi efficiently degrade the lignin in comparison to Ascomycetes and Deuteromycetes which oxidize phenolic compounds to give phenoxy radicals and quinines [43]. Basidiomycetes such as Phanerochaete chrysosporium, Theiophora terrestris, and Lenzites, betulina, and white-rot fungi such as Phlebia radiate, Pleurotus ostreatus, and Trametes versicolour (formerly known as Coriolus versicolor or Polyporus versicolor)also produce laccase. Trametes versicolor laccase has been applied in a number of processes ranging from baking to bioremediation as well as modification of polymers. Other laccase producers of Trametes include T. pubescens [44], T. hirsuta [45] and T. gallica [46]. Many Trichoderma species such as T. atroviride, T. harzianum, and T. longibrachiatum are the sources of laccases. Pleurotus ostreatus, for instance, belongs to a subclass of lignin–degrading microorganisms that produce laccase, manganese peroxidase and veratryl alcohol oxidase but no lignin peroxidase [47]. Pycnoporus cinnabarinus has been shown to produce laccase as ligninolytic enzyme [43] and Pycnoporus sanguineus produces laccase as phenol oxidase [16].
In fungi laccases have been implicated in many cellular processes, including delignification of lignocellulosic material, sporulation, pigment production, fungal morphogenesis, protection against toxic compounds, fruiting body formation and plant pathogenesis [4, 48]. Ligninolytic enzymes have mostly been reported to be extracellular but there is evidence in literature of the occurrence of intracellular laccases in white–rot fungi [49]. Fungal laccase isozymes are extracellular and intracellular types. Intracellular as well as extracellular laccases were identified for Neurospora crassa by Froehner and Eriksson [50], who suggested that the intracellular laccase functioned as a precursor for extracellular laccase as there were no differences between the two laccases other than their occurrence.
In plant-pathogenic fungi, laccases are important virulence factors. The grapevine grey mould, Botrytis cinerea, produces a laccase that is necessary for pathogenesis, and the role of the laccase is presumably related to detoxification of toxic defence metabolites produced by the plant [51]. Laccases have also been shown to be important for pathogenesis in the chestnut blight fungus Cryphonectria parasitica [52, 53] and in the human pathogen Cryptococcus neoformans [54]. In Aspergillus nidulans, laccase activity is related to pigment production, and deletion of the laccase gene yA abolishes the green color of conidial spores [55, 56]. Laccases have also been proposed to participate in fungal morphogenesis in Armillaria spp. [57], Lentinus edodes [58] and Volvariella volvacea [59].
Fungal laccases have higher redox potential than bacterial or plant laccases (up to +800 mV), and their action seems to be relevant in nature finding also important applications in biotechonology. Thus, fungal laccases are involved in the degradation of lignin or in the removal of potentially toxic phenols arising during lignin degradation [4]. In addition, fungal laccases are hypothesized to take part in the synthesis of dihydroxynaphthalene melanins, darkly pigmented polymers that organisms produce against environmental stress [60] or in fungal morphogenesis by catalysing the formation of extracellular pigments [61]. Laccase of the animal pathogen Cryptococcus neoformans oxidizes dihydroxyphenylalanine into a melanin-like pigment [62, 63]. Aspergillus nidulans laccase is required for pigment biosynthesis during conidial development and maturation [64].
Bacteria
The first bacterial laccase was detected in the plant root-associated bacterium Azospirillum lipoferum [65], where laccase was associated with the melanin production for cell pigmentation[66]. Recently some bacterial laccases have also been characterized from Azospirillum lipoferum, Bacillus subtilis, Streptomyces lavendulae, S. cyaneus and Marinomonas mediterranea.
A number of roles for laccases in bacterial systems have been suggested and include roles in melanin production, spore coat resistance against hydrogen peroxide and UV [67, 68] and involvement in morphogenesis [69]. An atypical laccase containing six putative copper-binding sites was discovered from Marinomonas mediterranea, but no functional role has been assigned to this enzyme [70, 71]. Bacillus subtilis produces a thermostable CotA laccase which participates in pigment production in the endospore coat [72]. In Streptomyces cyaneus, a laccase-type phenol oxidase was found to be produced during growth under solid-substrate fermentation conditions, and it was suggested that this enzyme was involved in the solubilization and mineralization of lignin from wheat straw [73]. Further studies demonstrated that this organism could be used to improve the qualities of pulp after 2 weeks of incubation under solid-substrate fermentation conditions [74]. However, until now there have been no reports describing the involvement of bacterial laccases in the oxidation of nonphenolic compounds in the presence of mediators. Characterization of bacterial laccases has revealed that they have a low redox potential (0.45-0.54 V) [75] but that they are active and stable at high temperatures (66 h at 60°C), pH (7-9) and salt concentrations [40, 67]. The application of bacterial laccases in dye decolorization and textile wastewater treatment confirms the industrial potential of these bacterial enzymes.
In addition to plants, fungi and bacteria, laccases or laccase-like activities have been found in some insects, where they have been suggested to be active in cuticle sclerotization [76, 77].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHYSICO-CHEMICAL PROPERTIES AND STRUCTURE
Laccases often occur as isoenzymes or monomers that oligomerize to form multimeric complexes. Each isoenzyme has four copper atoms and is able to individually carry on the catalytic mechanism of laccases. The molecular mass of the laccase monomers ranges from 40 to 130 kDa with a covalently linked carbohydrate content of 10–25% in fungi and 20-45% in plants. The carbohydrate moiety typically consists of mannose, N-acetylglucosamine and galactose, which may contribute to the high stability of the enzymes [78, 79].
The acidic isoelectric point of laccases is around pH 4.0. The pH optima of laccases are highly dependable on the substrate. Usually, fungal laccases exhibit pH optima in the range of 3-5 and bacterial laccases in the range of 5-6, when the substrate is a hydrogen atom donor compound (i.e., ABTS) [80]. When the substrate is a phenolic compound (e.g., syringaldazine), the optimal pH is shifted to 6-7. This shift in pH is a result of the balance of redox potentials between the substrate and the inhibition of the T2/T3 copper site by binding of an OH- ion.
The optimal temperature differs with the source of laccase, being 40 °C for Fusarium sp. BOL35 laccase, 50 °C for Trametes versicolor, 60 °C for Galerina sp HC1, and 70 °C for Myceliophthora thermophila. In general, laccases are stable at 30-50°C and rapidly lose activity at temperatures above 60°C [5]. The most thermostable laccases have been isolated from bacteria; the half-life of Streptomyces lavendulae laccase was 100 minutes at 70°C [81] and that of Bacillus subtilis CotA was 112 minutes at 80°C [72]. The typical half-lives of fungal laccases are clearly below one hour at 70°C and below 10 minutes at 80°C [5].
The redox potential ranges from 0.4-0.5 V in plants and bacteria to 0.4-0.9 in fungal laccases. The redox potential of the Type 1 site has been determined for many laccases using different mediators and varies from 430 mV for the laccase from R. vernicifera tree up to 780 mV for fungal laccase from Polyporus versicolor. It was previously found that the catalytic efficiency (kcat/Km) of laccases for some reducing substrates depended linearly on the redox potential of the Type 1 copper, in the sense that the higher the potential of the Type 1 site the higher the catalytic efficiency. That is why laccases with a high redox potential of the Type 1 site are of special interest in biotechnology, e.g., for efficient bleaching and bioremediation processes The catalytic action of an enzyme is quantitatively described by the Michaelis constant Km and the catalytic efficiency constant kcat. Km values are similar for the cosubstrate dissolved oxygen (about 5-10 M), but Vmax varies with the source of laccase (50–300 M/s). The turnover is heterogeneous over a broad range depending on the source of enzyme and substrate/type of reaction. The kinetic constants differ in their dependence on pH. Km is pH-independent for both substrate and co-substrate, while Kcat is pH-dependent.
Three-dimensional structural analysis of several fungal, bacterial and plant laccases reveals that all are composed of three sequentially arranged cuprodoxin-like domains; each of them with a greek key β-barrel topology, highly related to small copper proteins such as azurin and plastocyanin [40]. The multiple alignment of primary sequences of laccases shows that the copper binding motifs are highly conserved in all sequences, which reflects a common mechanism for copper oxidation and oxygen reduction [40]. However, putative binding pocket analysis reveals that bacterial laccases have larger binding cavities when compared to those from plants and fungi [40].
Until recently, the three-dimensional structure of five fungal laccases has been reported: Coprinus cinereus (in a copper Type 2-depleted form) [82], Trametes versicolor [83], Pycnoporus cinnabarinus [84], Melanocarpus albomyces [85] and Rigidoporus lignosus [86], the latter four enzymes with a full complement of Cu ions. Moreover, thethree-dimensional structure of the CoA laccase from Bacillus subtilis endospore hasalso recently been published [87, 88].
Laccases contain 4 copper atoms termed Cu T1 (where the reducing substrate binds) and trinuclear copper cluster T2/T3 (electron transfer from type I Cu to the type II Cu and type III Cu trinuclear cluster reduction of oxygen to water at the trinuclear cluster). These four copper ions are classified into three categories: Type 1 (T1), Type 2 (T2) and Type 3 (T3). These three types can be distinguished by using UV/visible absorption and electronic paramagnetic resonance (EPR) spectroscopy.
At oxidizing state, the Type 1 Cu (T1) gives blue colour to the protein at an absorbance of 600-610 nm which is EPR detectable. The T1 copper has a trigonal coordination with two histidines and one cysteine; in bacterial laccases the axial ligand is confirmed by methionine and in fungal laccase by leucine or phenylalanine. Type 2 Cu (T2) does not give colour but is EPR detectable and Type 3 Cu (T3) contains a pair of atoms in a binuclear conformation that give a weak absorbance in the near UV region but not detected by EPR signal, with an absorption band at 330 nm. The Type 2 copper and Type 3 copper form a trinuclear centre which is involved in the enzyme catalytic mechanism.
Spectroscopy combined with crystallography has provided a detailed description of the active site in laccase. Magnetic circular dichroism (MCD) and X-rays absorption spectroscopy of laccase have shown that the Type 2 and 3 centers combine to function as a trinuclear copper cluster with respect to exogenous ligand interaction including reaction with dioxygen. The Type 2 center is 3-coordinate with two histidine ligands and water as ligands. The Type 3 coppers are each 4-coordinate, having three histidines ligands and bridging hydroxide (Fig. 1). The structural model of bridging between the Type 2 and 3 has provided insight into the catalytic reduction of oxygen to water. It has been elucidated that the Type 2 copper is required for the reduction of oxygen since bridging to this center is involved in the stabilization of the peroxide intermediate. Reduction of oxygen by laccase appears to occur in two 2e− steps. The first is rate determining. In this Type 2/3 bridging mode for the first 2e− reduced, the peroxide-level intermediate would facilitate the second 2e− reduction (from the Type 2 and 1 centers) in that the peroxide is directly coordinated to reduced Type 2 copper, and the reduced Type 1 is coupled to the Type 3 by the covalent Cys–His linkages. It is clear that the Type 2 Cu is required for dioxygen reactivity in laccase and that dioxygen reduction occurs in the absence of the Type 1 Cu. This demonstrates that the Type 2/3 trinuclear Cu site represents the active site for the binding and multielectron reduction of dioxygen. The Type 1 Cu is clearly not necessary for reactivity with dioxygen.
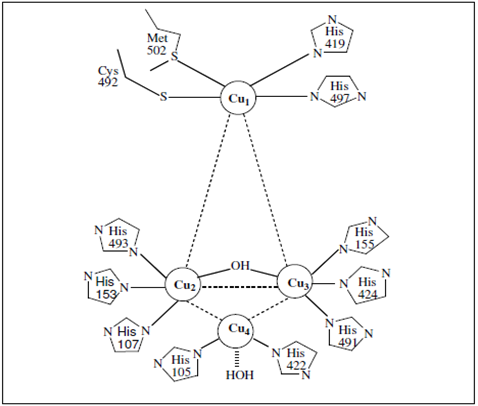
Figure 1 : The laccase active site showing the relative orientation of the copper atoms.The Type 1 copper is coordinate with two histidine ligands and two sulphurs one of methionine and the other of cysteine. The Type 2 center is 3-coordinate with two histidine ligands and water as ligands. The Type 3 coppers are each 4-coordinate, having three histidines ligands and bridging hydroxide [21, 87].
MODE OF ACTION OF LACCASE ENZYME
The laccase molecule is a dimeric or tetrameric glycoprotein, which usually contains four copper atoms per monomer distributed in three redox sites. The use of molecular oxygen as the oxidant and the fact that water is the onlyby-product are very attractive catalytic features, rendering laccases asexcellent ‘green’ catalysts [89].Laccases are particularly abundant in white-rot fungi, which are the only organisms able to degrade the whole wood components.
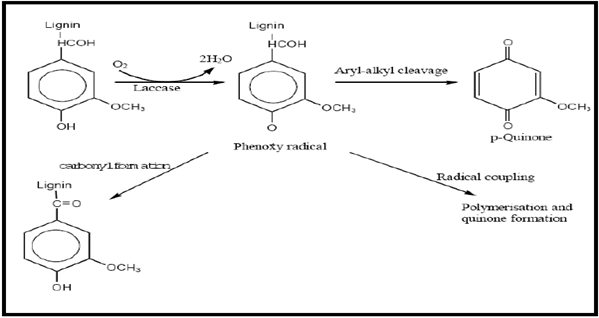
Figure 2 : Oxidation of phenolic compound (natural substrate) of lignin by laccase. Modified from Madhavi & Lele 2009[91].[R= lignin]
The natural substrates of laccase include phenols like ortho- and para-diphenols, aminophenols, polyphenols, polyamines and aryl diamines [90]. The oxidation of these molecules is represented in Figure 2. In general terms, substrate oxidation by laccase is a one-electron reaction generating a free radical. The initial product is typically unstable and may undergo a second enzyme-catalysed oxidation or otherwise a non-enzymatic reaction such as hydration, disproportionation or polymerization. These enzymes are polymeric and generally contain 1 each of type 1, type 2, and type 3 copper centre/subunit where the type 2 and type 3 are close together forming a trinuclear copper cluster. The bonds of the natural substrate, lignin, that are cleaved by laccase include, Cα- oxidation, Cα-Cβ cleavage and aryl-alkyl cleavage
Laccase mediator system
Laccase can oxidize only phenolic fragments of lignin due to the random polymer nature of lignin and to the laccase lower redox potential. Small natural low molecular weight compounds with high redox potential than laccase itself (> 900 mV) called mediators may be used to oxidize the non-phenolic part of lignin [43]. In the last years the discovery of new and efficient synthetic mediators extended the laccase catalysis towards xenobiotic substrates [43, 92, 93]. Laccases were thought to play a role in biodegradation of lignin, but is was restricted to phenolic compounds because of the low oxidation potentials of these enzymes. Application of these enzymes in the presence of mediator compounds resulted in high oxidation capability, leading to oxidation of non-phenolic lignin model compounds [Figure 3].
Figure 3 : Oxidation of non-phenolic lignin model compounds by a laccase mediator system.
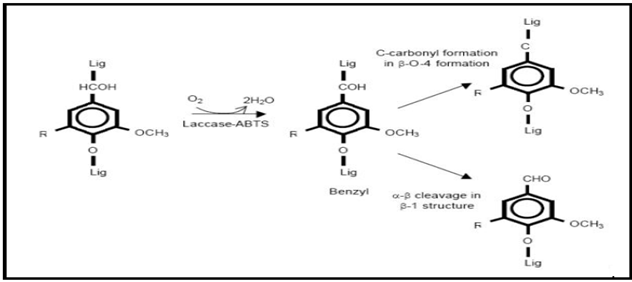
The laccase-mediated catalysis can be extended to nonphenolic substrates by the insertion of mediators. The highly active cation radicals oxidize the non-phenolic compounds that laccase alone cannot oxidize. Several organic and inorganic compounds have been reported as effective mediators. The diammonium salt 2,2'-azinobis (3- ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1-hydroxybenzotriazole (HBT), N-hydroxyacetanilide (NHA), violuric acid (VLA), N-hydroxyphthalimide (HPT) and 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) (and its derivatives) are act as Synthetic mediators. In presence of ABTS oxygen uptake by laccase is faster than the HBT. The application of these mediators can be limited due to their high cost as well as their toxicity.
On the other hand, molecules like vanillin, p-coumaric acid, acetovanillone, methyl vanillate, syringaldehyde, acetosyringone and some dyes are reported as “natural” mediators. They have been shown to catalyze reactions as effectively as the other type of mediators. Additionally, they can be easily produced from lignin. The LMS of successfully applied to the oxidation of aromatic methyl groups, benzyl alcohols, polycyclic aromatic hydrocarbons (PAHs) and bleaching of textile dyes.
From a mechanistic point of view, the reactions catalysed by laccases can be represented by one of the schemes shown in Figure 4. The simplest case (Figure 4(a)) is the one in which the substrate molecules are oxidised to the corresponding radicals by direct interaction with the copper cluster. However, the substrates of interest cannot be oxidised directly by laccases, either because they are too large to penetrate into the enzyme active site or because they have a particularly high redox potential. By mimicking nature, it is possible to overcome this limitation with the addition of “redox mediators”, that act as intermediate substrates for laccases, whose oxidised radical forms are able to interact with the bulky or high redox potential substrate targets (Figure 4(b)).
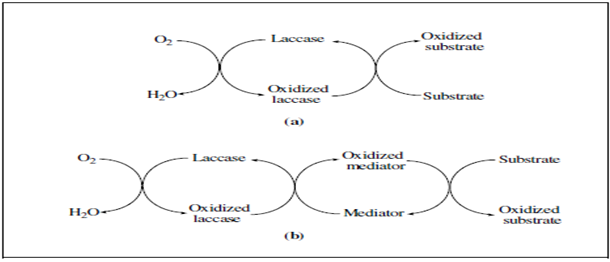
Figure 4 :Schematic representation of laccase-catalysed redox cycles for substrates oxidation in the absence (a) or in the presence (b) of redox mediators [89].
MOLECULAR BIOLOGY OF LACCASES
The first gene and cDNA sequences were recorded for laccase from the fungi Neurospora crassa , Aspergillus nidulans, Coriolus hirsutus and Phlebia radiate. Since then, the number of laccase genes sequenced has increased considerably, and searches from protein and gene sequence databases currently yield several hundreds of laccase gene sequences. However, a significant number of these are only partial stretches of putative laccase genes that have been found in genome-wide sequencing projects and have been annotated on the basis of sequence homology with known laccases. The number of laccase genes of which the corresponding protein products have been experimentally characterized is significantly lower. To date, there are about 21 such enzymes, most of which are fungal laccases (Table 1). In addition to the genes shown in Table 1, several laccase genes have been characterized in detail at the nucleotide level but have not been specified to code for a known laccase protein.
The sequences mostly encode polypeptides of approximately 500 to 600 amino acids (including the N-terminal secretion peptide). All the laccases listed in Table are secreted proteins, and typical eukaryotic signal peptide sequences of about 21 amino acids are found at the N-termini of the protein sequences. In addition to the secretion signal sequence, laccase genes from N. crassa, P. anserina, M. thermophila and C. cinereus contain regions that code for N-terminal cleavable propeptides. These laccases also have C-terminal extensions of controversial function, i.e. the last amino acids from the predicted amino acid sequence are not present in the mature protein.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table1 : laccase genes to encode a biochemically characterized laccase protein.
|
Organism |
Gene |
Protein encoded by the gene |
Reference |
|||
|
Name |
EMBL Acc.No. |
Length (aa) |
MW (kDa) |
pI |
||
|
Bacillus subtilis |
cotA |
U51115 |
513 |
65 |
7.7 |
Martins et al. 2002 [72] |
|
Botrytis cinerea |
Bclcc2 |
AF243855 |
581 |
_ |
_ |
Schouten et al. [98] |
|
Ceriporiopsis subvermispora |
lcs-1 |
AY219235 |
519 |
79 |
3.6 |
Salas et al. 1995 [99]; Karahanian et al. 1998 [100] |
|
Coprinus cinereus |
lcc1 |
AF118267 |
539 |
63 |
3.7-4.0 |
Yaver et al. 1999 [101] |
|
Cryptococcus neoformans |
CNLAC1 |
L22866 |
624 |
75 |
_ |
Williamson 1994 [54] |
|
Gaeumannomyces graminis var. tritici |
LAC2 |
AJ417686 |
577 |
_ |
_ |
Edens et al. 1999 [102] |
|
Marasmius quercophilus (Basidiomycete C30) |
lac1 |
AF162785 |
517 |
62 |
3.6 |
Dedeyan et al. 2000 [103] |
|
Melanocarpus albomyces |
Lac 1 |
AJ57169 |
623 |
_ |
_ |
Kruus et al. [104] |
|
Myceliophthora thermophila |
lcc1 |
AR023901 |
619 |
80 |
4.2 |
Berka et al. 1997a & b [105, 106] |
|
Neurospora crassa |
2 alleles |
M18333 - 4 |
619 |
64 |
6.8 |
Germann et al.1988[107] |
|
Phlebia radiate |
lac1 |
X52134 |
548 |
64 |
3.5 |
Niku-Paavola et al. 1990 [108] |
|
Pleurotus ostreatus |
poxa1b |
AJ005017 |
533 |
62 |
6.9 |
Giardina et al. 1999[109] |
|
Pleurotus ostreatus |
poxc (=pox2) |
Z49075 |
533 |
67 |
4.7 |
Palmieri et al. 1993[110]
|
|
Basidiomycete PM1 (CECT 2971) |
lac1 |
Z12156 |
517 |
64 |
3.6 |
Coll et al. 1993a & b [111, 112] |
|
Podospora anserina |
lac2 |
Y08827 |
621 |
70 |
7-10 |
Fernández-Larrea and Stahl 1996 [113] |
|
Populus euramericana |
lac90 |
Y13772 |
574 |
90 |
9.2 |
Ranocha et al. 1999 [31] |
|
Rhizoctonia solani |
lcc4 |
Z54277 |
530 |
66 |
7.5 |
Wahleithner et al. 1996 [114] |
|
Streptomyces lavendulae |
_ |
AB092576 |
631 |
73 |
_ |
Suzuki et al. 2003 [81] |
|
Trametes pubescens |
lap2 |
AF414807 |
523 |
65 |
2.6 |
Galhaup et al. 2002 [115] |
|
Trametes trogii |
lcc1 |
Y18012 |
496 |
70 |
3.3-3.6 |
Colao et al. 2003 [116] |
|
Trametes versicolor |
lccI |
L49376 |
519 |
67 |
_ |
Ong et al. 1997 [117] |
|
Trametes versicolor |
lcc2 |
U44430 |
520 |
64 |
3.1-3.3 |
Cassland and Jönsson 1999 [118] |
|
Trametes villosa |
lccI |
L49377 |
520 |
63 |
3.5 |
Yaver et al. 1996 [119] |
|
Trametes villosa |
lcc2 |
AY249052 |
519 |
63 |
6.2-6.8 |
Yaver et al. 1996 [119] |
The molecular weights of laccases are usually in the range of 60 to 90 kDa when determined by SDS-PAGE. The difference between the molecular weight (MW) predicted from the peptide sequence and the experimentally obtained MW is caused by glycosylation, which typically accounts for about 10.20% of the total MW. The isoelectric points of microbial laccases are generally around 3.6. However, many laccase-producing fungi produce several laccase isoforms, and laccases with pIs at neutral or slightly alkaline pH values have also been detected in several fungi, such as Podospora anserina, Rhizoctonia solani, Trametes villosa and Pleurotus ostreatus.
Many fungal genomes contain more than one laccase gene. Trametes villosa, for example, contains at least five laccase genes, Coprinus cinereus at least eight and Rhizoctonia solani, Pleurotus sajor-caju and Pleurotus ostreatus at least four laccase genes. The precise quantification of laccase genes is complicated by the existence of different laccase gene alleles in the chromosomes, because most of the studied laccase-producing fungi are diploid. Laccase proteins and thereby also laccase genes are identified by the presence of four highly conserved copper binding motifs, all involving the sequence HXH and containing altogether 10 conserved histidines and one conserved cysteine. These copper binding regions can also be found in other multicopper oxidases, which complicates the identification of laccase genes without knowledge of the properties of the corresponding protein. For example, fungal ferroxidase from Phanerochaete chrysosporium has been shown to be about 30% identical to fungal laccases, and it contains the same conserved copper binding residues as laccases.
The transcriptional induction of laccase genes by metal ions and phenolic compounds has been suggested to result from the presence of specific regulatory sites in the promoter regions of the genes. The upstream regulatory regions of several laccase genes have been shown to contain putative metal-responsive elements (MRE) that have also been found in promoter regions of metallothionein proteins involved in metal homeostasis and detoxification. Furthermore, putative heat-shock elements (HSE), xenobiotic response elements (XRE) and antioxidant response elements (ARE) have been discovered from the promoter regions of laccase genes, although the roles of these regulatory regions have not yet been experimentally demonstrated.
Comparison of laccase gene nucleotide sequences indicates that laccases can be divided into at least three different groups: basidiomycete, ascomycete and plant laccases [94, 95]. The level of amino acid identity between laccases from the same group is generally above 50%, whereas identity between laccases from different groups is below 40%. The translated laccase genes yA and tilA from the ascomycete Aspergillus nidulans differ significantly from other laccase protein sequences [96, 97]; the level of amino acid identity between the predicted Aspergillus laccases and other laccases is only about 30% based on BLAST similarity searches. The bacterial laccase proteins from Bacillus subtilis [72] and Streptomyces lavendulae [81] are 47% similar to each other but differ very much from other laccases. Pairwise similarity between the bacterial and fungal laccase proteins is less than 30%. The similarity of bacterial laccases is actually higher with other bacterial multicopper proteins, such as Streptomyces antibioticus phenoxazinone synthase and Escherichia coli copper homeostasis protein CueO, than with other laccases.
LACCASE PRODUCTION
Laccases are the enzymes which are secreted out in the medium extracellularey by several fungi during the secondary metabolism. The demand for laccase for different applications requires the production of the enzyme in large amounts. Gene expression, the modification of culture conditions, or a combination of both strategies are utilized to cover the demand.
Table 2 : Laccase production in heterologous hosts.
|
Laccase gene |
Production host |
Laccase Production (mg/l) |
Reference |
|
Ceriporiopsis subvermispora lcs-1 |
Aspergillus nidulans Aspergillus niger |
1.5 1.5 |
Larrondo et al. 2003 [121] Larrondo et al. 2003 [121] |
|
Coprinus cinereus lcc1 |
Aspergillus oryzae |
135 |
Yaver et al. 1999 [101] |
|
Melanocarpus albomyces lac1 |
Trichoderma reesei |
920 |
Kiiskinen et al. [122] |
|
Myceliophthora thermophila lcc1 |
Aspergillus oryzae Saccharomyces cerevisiae |
19 18 |
Berka et al. 1997b [106] Bulter et al. 2003 [123] |
|
Phlebia radiate lac1 |
Trichoderma reesei |
20 |
Saloheimo and Niku-Paavola 1991 [124] |
|
Pleurotus sajor-caju lac4 |
Pichia pastoris |
4.9 |
Soden et al. 2002 [125] |
|
Pycnoporus cinnabarinus lac1 |
Pichia pastoris Aspergillus niger Aspergillus oryzae Schizophyllum commune |
8 70 80 1200 |
Otterbein et al. 2000 [126] Record et al. 2002 [127] Sigoillot et al. 2004 [128] Alves et al. 2004 [129] |
* The reported production levels have been obtained in shake flask cultivations, except in the case of Phlebia radiata laccase which was produced in a laboratory fermentor.
· Heterologous production of laccases
Heterologous expression of the genes encoding the laccases is carried out in order to increase their production and to be able to apply them in large-scale processes/applications. However, the expression of laccase genes in different hosts has not shown any significant enhancement in the production up until now. Nevertheless, heterologous production can help in the characterization of individual laccase isoenzymes [101] as well as in avoiding additional production of toxic compounds besides the laccase.
Laccase genes are often expressed at very low levels in the native hosts. In order to improve laccase production, fungal laccases have been expressed heterologously in Saccharomyces cerevisiae, Trichoderma reesei, Aspergillus oryzae, Pichia pastoris, Yarrowia lipolitica, Aspergillus sojae, Aspergillus niger, Aspergillus nidulans, Aspergillus ficuum, tobacco and maize. Bacterial laccases from B. subtilis, Thermus thermophilus and Streptomyces lavendulae have been expressed in Escherichia coli but successful expression of fungal laccases in E. coli has not been reported.
In spite of the fact that laccase production levels have often been improved significantly by expression in heterologous hosts, the reported levels are still rather low for industrial applications (Table 2). Improved laccase production levels have also been achieved by expression in P. pastoris, whereas expression in S. cerevisiae has generally resulted in very low activity levels. The addition of copper into the culture medium also proved to be important for heterologous laccase production in P. pastoris and Aspergillus spp. [120].
· Cultivation parameters for laccase production
* Influence of Carbon and Nitrogen Source
The organism grown in the defined medium contains 0.1%w/v yeast extract and 1% (w/v) different carbon sources as well as nitrogen sources. Glucose, mannose, maltose, sucrose, fructose, cellobiose, cellulose, glycerol and lactose are the commonly used carbon sources. Fructose was shown to be a good carbon source for laccase production in Pleurotus sajor-caju, cellobiose in T. pubescens, and lactose or glycerol in Pseudotrametes gibbosa, Coriolus versicolor and Fomes fomentarius. The use of excessive concentrations of glucose and sucrose as carbon source in cultivation of laccase producing fungal strains has an inhibitory effect on laccase production. This problem of production of enzyme can be improved by using polymeric substrates like cellulose as carbon source during cultivation.
Yeast extract, peptone, urea, (NH4)2SO4, and NaNO3 are the commonly used nitrogen sources. Laccase production is triggered by nitrogen depletion but some nitrogen strains do not affect the enzyme activity. Low nitrogen levels (as yeast extract) improves the laccase production in Pleurotus ostreatus, Coriolus versicolor and Pycnoporus sanguineus, high concentrations are needed for Trametes pubescens, Trametes gallica and Galerina sp. HC1. Some studies show that the elevated laccase activity was achieved by using low carbon-to-nitrogen ratio while others show that it was achieved at high carbon-to-nitrogen ratio.
Casein, another nitrogen source was successfully used for the production of laccase in Pleurotus sajor-caju. In Trametes versicolor and Coriolopsis polyzona the laccase production was significantly improved when NH4NO3, (NH4)2SO4, KNO3 and peptone were used as supplementary nitrogen sources. On the other hand, agricultural residues can be used as carbon and nitrogen sources for laccase production. Cultivation of Galerina sp. HC1 on bagasse and orange peels demonstrated the potential of the agro residues as substrates for laccase production: the production was increased 1- and 4-fold, respectively when compared to cultivation in glucose containing medium.
* Induction of laccase production
Laccase production has been found to be highly dependent on the conditions for the fungus cultivation and media supporting high biomass did not necessarily support high laccase yields. Laccases were generally produced in low concentrations by laccase producing fungi, but higher concentrations were obtainable with the addition of various supplements to media. The addition of aromatic compounds such as 2,5-xylidine, lignin, and veratryl alcohol is known to increase and induce laccase activity. Many of these compounds resemble lignin molecules or other phenolic chemicals. Veratryl alcohol is an aromatic compound known to play an important role in the synthesis and degradation of lignin. The addition of veratryl alcohol to cultivation media of many white-rot fungi has resulted in an increase in laccase production. Some of these compounds affect the metabolism or growth rate while others, such as ethanol, indirectly trigger laccase production.
Eggert et al. [43] found that the addition of 2,5-xylidine as inducer had the most pronounced effect on laccase production. The addition of 10 μM 2,5-xylidine after 24 h of cultivation gave the highest induction of laccase activity and increased laccase activity nine-fold [43]. At higher concentrations the 2,5-xylidine had a reduced effect, probably due to toxicity [43]. Osma et al. [130] showed that soya oil was the best inducer of laccase activities, attaining 4- fold higher than those obtained in the reference cultures.
Copper can have a toxic effect in many organisms [131]. However, the addition of low concentrations of Cu+2 to the cultivation media of laccase producing fungi stimulates laccase production.The growth of Galerina sp. HC1 was highly favored when 0.01 g/l copper sulphate was added to the cultivation. Palmieri et al. [47] found that the addition of 150 μM copper sulphate to the cultivation media can result in a fifty-fold increase in laccase activity compared to a basal medium. Employing copper sulphate as laccase inducer or supplementing the culture medium with veratryl alcohol, led to maximum values of laccase activity. A new basidiomycete, Trametes sp. 420, produced laccase in glucose medium and in cellobiose medium with induction by 0.5 mM Cu+2 and 6 mM o-toluidine.
* Additives
The addition of chemicals to the cultivation can enhance the laccase production based on their ability to induce the expression of thedifferent isoforms. p-Coumaric acid, α?benzoin oxime and 2,5-xylidine highly improved laccase production in Galerina sp. HC1, whereas lignin or lignin-related structures only enhanced the production slightly. Other lignin-related chemicals such as ferulic acid or vanillin proved to increase the laccase production up to 10 times in Pleurotus pulmonarius; vanillin induced the production in Phanerochaete flavido-albaand caffeic acid in Coprinus comatus. Vitamins like biotin, riboflavin and pyridoxine hydrochloride as well as amino acids such as methionine, tryptophan, glycine and valine stimulated laccase production in Cyathus bulleri, whereas cysteine inhibited the production. Antibiotics like apramycin sulfate stimulated laccaseproduction in Cyathus bulleri and Pycnoporus cinnabarinus. Metals like Mn2+ led to a 4.5-fold increase in the laccase productionby Coprinus comatus [132].
* Operational conditions
Other important parameters include the temperature, pH, aeration level and agitationduring the cultivation. Most reports indicate that an initial pH of 4.5-6.0 and a temperature between 25-30 °C are suitable for laccase production. Nyanhongo et al. [133] reported that an initial pH of 7.0 was the best for optimal growth and laccase production by a newly isolated strain of T. modesta.However, the effect of aeration varies between species; growth of some fungi is highly favored with aeration, while others can suffer from stress caused by oxygen. In addition, as aeration can involve mechanical stirring, this may cause stress on the cells by rupturing them. Agitation is another factor which affects laccase production. Hess et al. [134] found that mycelia are damaged when fungus is grown in the stirred tank reactor and laccase production by Trametes multicolour is considerably decreased. Tavares et al. [135] observed that agitation did not play any role in the production of laccase by T. versicolor.
· Type of Cultivation
Submerged and solid-state modes of fermentation are used intensely for the production of laccase. Wild-type filamentous fungi are used for large-scale production of laccase in different cultivation techniques.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
* Submerged cultivation
The process of submerged cultivation involves the growth of microorganisms in a liquid medium rich in nutrients under aerobic conditions. In order to achieve high production, the studies are first focused on the optimization of nutritional and operational conditions [46]. Galerina sp. HC1 produced high laccase activity under optimizedconditions in batch submerged fermentation.Submerged cultivation can be carried out by utilizing cheap materialsconsidered as “waste” and which are produced in large amounts. Thesematerials can contain considerable concentrations of soluble carbohydrates,nitrogen, minerals and vitamins, and even inducers for enzyme production(Table 3).
The industrial production of enzymes is mainly achieved by submerged cultivation. One of the disadvantages of this method is the excessive growth of mycelium, which affects the production yield due to mass transfer and metabolic rate limitation. The excessive growth can also affect the mechanical set up of the used reactor. In other words, the mycelia can wrap around the impellers, cause blockage of the system and increase viscosity; however, this has been overcome by immobilization of the cells in various supports (Table 3) or by using fed-batch cultivation for controlling the fungal growth [115].
* Solid state fermentation
Solid state fermentation (SSF) is defined as a cultivation technique where additional liquid can be nearly or totally absent. During the cultivation, synthetic or natural substrates can be used as the solid support (Table 4). A possible reason for the high level of enzyme production obtained by SSF is that it “simulates” the conditions of natural growth for fungi. In addition, the low cost of the substrates, reduced risk of bacterial contamination, low energy requirements as well as downstream processing of the enzyme are considered as the principal advantages of implementing this type of cultivation.
The principal disadvantages for growing microorganisms in SSF are related to the low transfer of oxygen, nutrients, moisture, temperature and regulation of pH [136]. However, the magnitude of the disadvantage will greatly depend on the type of reactor and the characteristics of the substrate used (Table 4) [45].
Table 3 : Production of laccase in submerged cultivation using agricultural and synthetic materials.
|
Source |
Support |
Production (U/l) |
Reactor |
Reference |
|
Galerina sp. HCl |
Orange peels Bagasse |
2000 600 |
Batch |
Laura Mendoza et al. 2010 [137] |
|
Ganoderma adspersum |
Corn bran, soy bran, chicken feathers, wheat bran, kiwi |
600-3400 |
Batch |
Songulashvili et al. 2006 [138] |
|
Lentinus edodes |
Malted barley (brewing process) |
100 |
Batch |
Hatvani & Mécs 2001 [139] |
|
Neurospora crassa |
Capillary membrane supports |
10000 |
Batch |
Rodríguez Couto & Herrera 2007 [136] |
|
Phellinus robustus |
fruits, banana peels, mandarin peels, ethanol production residue and cotton stalks |
700-4000 |
Batch |
Songulashvili et al. 2006 [138] |
|
Pleurotus eryngii |
Dried ground mandarin peels |
999 |
Batch |
Staji? et al. 2006 [140] |
|
Pleurotus ostreatus |
Immobilized in polyurethane foam |
1403 |
Packed bed reactor |
Rodríguez Couto & Herrera 2007 [136] |
|
Pycnoporus cinnabarinus |
Cubes of sponge |
280 |
Packed bed reactor |
Rodríguez Couto & Herrera 2007 [136] |
|
Trametes hirsuta |
Alginate beads |
1043 |
Air lift reactor |
Rodríguez Couto & Herrera 2007 [136] |
|
Trametes versicolor (CBS100.29) |
Grape seeds, grape stalks and barley bran |
200-600 |
Batch |
Lorenzo et al. 2002 [141] |
The lignin, cellulose and hemicelluloses are rich in sugar and promote fungal growth in fermentor and make the process more economical [136]. Different bioreactor configurations have been studied for laccase production such as immersion configuration, expanded bed, tray, inert (nylon) and noninert support (barley bran) in which tray configuration gave the best response. A tray and immersion configuration is compared for laccase production by using grape seeds and orange peel as substrate.
Laccase production by both solid-state and submerged fermentation is higher in case of rice bran than other substrates. The rice bran inductive capability is based on the phenolic compounds such as ferulic acid, and vanillic acid which induce the laccase production. Many agricultural wastes such as grape seeds, grape stalks, barley bran, cotton stalk, molasses waste water and wheat bran are also used as substrate for laccase production. However, laccase production in both solid-state and submerged fermentation did not reach up to the maximum level; that is why prolonged cultivation is required.
· Purification of Laccase
In general, plant laccases are purified from sap or tissue extract, whereas extracellular fungal laccases are purified from culture medium (fermentation broth) of the selected organism. Various protein purification techniques are frequently used in purifying laccase. Typical purification protocol involve ultrafiltration, ion exchange, gel filtration, hydrophobic interaction, or other electrophoretic and chromatographic techniques. Affinity chromatography using a phenolic group as ligand can increased purification efficiency. The purity of laccase preparation is often measured by SDS-PAGE and by the ratio of the absorbance at 280 nm to that at 600 nm.
Table 4 : Production of laccase under solid state fermentation
|
Source |
Support |
Reactor |
Production (U/l) |
Reference |
|
Galerina sp. HCl |
Orange peels |
Cotton plugged Erlenmeyer flasks |
720 |
Laura Mendoza et al. 2010 [137] |
|
Pycnoporus cinnabarinus |
Sugarcane bagasse |
Packed reactor |
10000 |
Meza et al. 2006 [142] |
|
Trametes hirsuta |
Grape seeds |
Tray |
18715 |
Rodríguez Couto et al. 2006 [90] |
|
Trametes hirsuta |
Kiwi fruits |
Cotton plugged Erlenmeyer flasks |
5399 |
Rosales et al. 2005 [143] |
|
Trametes hirsuta |
Nylon sponge |
Tray |
6898 |
Rodríguez Couto et al. 2006 [90] |
|
Trametes hirsuta |
Orange peels |
Tray |
12000 |
Rosales et al. 2007 [144] |
|
Trametes pubescens |
Banana skin |
Cotton plugged Erlenmeyer flasks |
1570 |
Osma et al. 2007 [130] |
|
Trametes versicolor |
Barley bran |
Immersion |
600 |
Rodríguez Couto et al. 2003 [45] |
|
Trametes versicolor |
Barley bran |
Tray |
3500 |
Rodríguez Couto et al. 2003 [45] |
|
Trametes versicolor |
Nylon sponge |
Expanded bed |
229 |
Rodríguez Couto et al. 2003 [45] |
|
Trametes versicolor |
Nylon sponge |
Immersion |
229 |
Rodríguez Couto et al. 2003 [45] |
|
Trametes versicolor |
Nylon sponge |
Tray |
343 |
Rodríguez Couto et al. 2003 [45] |
Ammonium sulphate is being commonly used for the enzyme purification for many years. But researchers have found much more efficient methodologies such as protein precipitation by ammonium sulphate, anion exchange chromatography, desalt/buffer exchange of protein, and gel filtration chromatography. Single-step laccase purification from Neurospora crassa takes place by using celite chromatography and 54 fold purification was obtained with specific activity of 333 U mg−1 [145]. Laccase from LLP13 was first purified with column chromatography and then purified with gel filtration. Laccase from T. versicolour is purified by using ethanol precipitation, DEAE-Sepharose, Phenyl- Sepharose and Sephadex G-100 chromatography which is a single monomeric laccase with a specific activity of 91,443Umg−1 [134]. Laccase from T. versicolour is purified with Ion Exchange chromatography followed by gel filtration with specific activity of 101UmL−1 and 34.8-fold purification[146]. Laccase from Stereum ostrea is purified with ammonium sulphate followed by Sephadex G-100 column chromatography with 70-fold purification. Laccase from fruiting bodies is purified with ammonium sulphate precipitation with 40–70% saturation and DEAE cellulose chromatography then 1.34 and 3.07 fold purification is obtained respectively [147].
LACCASE IMMOBILIZATION
Enzymes exhibit a number of features that make their use advantageous as compared to conventional chemical catalysts. However, a number of practical problems exist that reduce their operational life-time, such as their high cost of isolation and purification, their non-reusability, the instability of their structures and their sensitivity to harsh process conditions. Many of these undesirable limitations may be overcome by the use of immobilized enzymes [148]. Immobilization is achieved by fixing enzymes to or within solid supports, as a result of which heterogeneous immobilized enzyme systems are obtained.
When enzyme is immobilized, it becomes more vigorous and resistant to alteration in environment which allows easy recovery and reuse of enzyme for multiple purposes. Compared with the free enzyme, the immobilized enzyme has usually its activity lowered and the Michaelis constant increased. These alterations result from structural changes introduced to the enzyme by the applied immobilization procedure and from the creation of a microenvironment in which the enzyme works, different from the bulk solution. That is why researchers are moving towards the efficient methods of immobilization which influence the properties of the biocatalys. Laccase immobilization has been studied with a wide range of different immobilization methods and substrates.
Enzymes may be immobilized by a variety of methods (adsorption, entrapment, crosslinking and covalent bonding) mainly based on chemical and/or physical mechanisms. The adsorption of chromophoric-oxidized products on the surface of the immobilizationsupport often leads to enzyme inactivation phenomena.Laccase produced by Trametes versicolour is immobilized on silica which is chemically modified with imidazole groups and Amberlite IRA-400. Glass-ceramic is chemically modified by carbodiimide/glutaraldehyde as well as aminopropropyltriethoxysilane/glutaraldehyde, and montmorillonite is modified by aminopropyltriethoxysilane/glutaraldehyde which was used in the decolorization of textile dyes [149]. Laccase can be immobilized on different pyrolytic graphite (best support), ceramics supports and on a carbon fiber electrode where it acts as biosensor. An optical biosensor is fabricated by using stacked films for the detection of phenolic compounds; 3-methyl-2-benzothiazolinone hydrazone (MBTH) was immobilized on a silicate film and laccase on a chitosan film.
Enzymatic catalysis in organic solvents has opened a new field of biotechnological applications of enzymes. As well in the presence of organic solvents there is less risk of microbial contamination. In the specific case of laccases or LMS, many of their non-natural substrate are hardly soluble in water (their Km values are far away from their solubility in aqueous media), and therefore the use of cosolvents is an indispensable requirement. However, the laccase may be denatured or it may be inhibited under these conditions. In this regard laccases can be applied in nonaqueous solution or multiphasic systems. For water-immiscible organic solvents, laccase may be entrapped in reverse micelles or immobilized onto a carrier [3, 5]. Preferably, the reverse micelles are used in the presence of laccase mediators to enhance and mediate the laccase activity in organic solvents.
LACCASE APPLICATION
A few laccases are at present in the market for textile, food and other industries (Table 5), and more candidates are being actively developed for future commercialization [79]. Laccase is important because it oxidizes both the toxic and nontoxic substrates. Laccases have been used in a variety of industrial and environmental applications since they can oxidize a number of natural and synthetic substrates using oxygen, producing water as the only by-product. Laccases are versatile enzymes able to oxidize recalcitrant compounds like lignin, which makes them attractive for use in various biotechnological processes. A vast amount of industrial applications for laccases have been proposed. This enzyme is very specific, ecologically sustainable and a proficient catalyst. Applications of laccases are as follows.
* Food Processing Industry
Laccase application in the food industry is based on its ability to polymerize molecules (Table 6) Laccases can be applied to certain processes that enhance or modify the colour appearance of food or beverage. In this way, laccase is used for the elimination of undesirable phenolic compound, responsible for the browning, haze formation and turbidity development in clear fruit juice, beer and wine. Laccases are currently of interest in baking due to its ability to cross-link biopolymers. Thus, Selinheimo et al. (2006)showed that a laccase from the white-rot fungus Trametes hirsuta increased the maximum resistance of dough and decreased the dough extensibility in both flour and gluten dough [150]. Alternatively, laccase participates in the oxidation of residual molecules produced during the processing of food [153].
Table 5 : Commercial preparations based on laccases for industrial processes.
|
Industry |
Main application |
Brand name |
Manufacturer |
|
Food industry
|
Brewing |
Flavourstar |
Advanced Enzyme Technologies Ltd. (India) |
|
Colour enhancement in tea, etc. |
LACCASE Y120 |
Amano Enzyme USA Co. Ltd. |
|
|
Cork modification |
Suberase |
Novozymes (Denmark) |
|
|
Paper industry |
Pulp bleaching |
Lignozym-process |
Lignozym GmbH (Germany) |
|
Paper pulp delignification |
Novozym 51003 |
Novozymes (Denmark) |
|
|
Textile Industry |
Denim bleaching |
Bleach Cut 3-S |
Season Chemicals (China) |
|
DeniLite |
Novozymes (Denmark) |
||
|
ZyLite |
Zytex Pvt. Ltd. (India) |
||
|
Denim finishing |
Cololacc BB |
Colotex Biotechnology Co. Ltd. (Hong Kong) |
|
|
Ecostone LC10 |
AB Enzymes GmbH (Germany) |
||
|
IndiStar |
Genencor Inc. (Rochester, USA) |
||
|
Novoprime Base 268 |
Novozymes (Denmark) |
||
|
Denim bleaching and shading |
Primagreen Ecofade LT100 |
Genencor Inc. (Rochester, USA) |
Table 6 : Application of laccase in the food industry for improvement of the quality of the products
|
Industry |
Substrate |
Improved parameter |
Reference |
|
Wine, Juices, Beer |
Phenolic compounds |
Clarification, aroma, flavor and taste |
Minussi et al. 2007 [152] |
|
Ripe-olive processing |
Phenolic compounds |
Debittering and darkening |
Xu 2005 [6] |
|
Sugar beet pectin gelation |
Feruloyl groups |
Thermostability of the gel |
Minussi et al. 2002 [151] |
|
Bakery |
Probably on glutenins, gliadins and arabinoxylans
|
Softness, strength, machinability of the dough |
Minussi et al. 2002 [151] ; Rodríguez Couto & Toca- Herrera 2006 [90] |
* Pulp and paper industry
Chlorine and oxygen-based chemical oxidants are used for the separation and degradation of lignin in wood pulp which is required for the preparation of paper at industrial level. Oxygen delignification process has been industrially introduced in the last years to replace conventional and polluting chlorine-based methods. In spite of this new method, the pre-treatments of wood pulp with laccase can provide milder and cleaner strategies of delignification that also respect the integrity of cellulose. Laccases are able to delignify pulp when they are used together with mediators [92]. Mediators may be used to oxidize the non-phenolic residues from the oxygen delignification [92]. The mediator is oxidized by laccase and the oxidized mediator molecule further oxidizes subunits of lignin that otherwise would not be laccase substrates.
Although the LMS has been studied extensively, But some problems such as mediator recycling, cost, and toxicity remain unsolved. However, some environmental benefits are envisaged and the fact that LMS could be easily implemented in the existing bleaching process because it lead to a partial replacement of ClO2 in pulp mills. Furthermore, the application of laccases in pulp-kraft bleaching may result in higher pulp yields and energy savings. Most of studies have been patented about the use of LMS in the pulp-kraft bleaching processes. On the other hand, laccase mediator systems can also be applied to remove pitch and dyes from wood-based materials. More recently, the potential of this enzyme for cross-linking and functionalizing ligninaceous compounds was discovered. In another related application, laccases can be even used for deinking and decolorizing a printed paper. Finally, laccases can be used for binding fiber-, particle- and paper-boards. However, different wood-decaying basidiomycetes have shown a highly variable pattern of laccase formation, and this subject requires more detailed experiments [53].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
* Textile industry
The textile industry accounts for two-thirds of the total dyestuff market and consumes large volumes of water and chemicals for wet processing of textiles. The chemical reagents used are very diverse in chemical composition, ranging from inorganic compounds to polymers and organic products.There are more than 100,000 commercially available dyeswith over 7×105 tonnes of dyestuff produced annually. Due to their chemical structure dyes are resistant to fading on exposure to light, water and different chemicals and most of them are difficult to decolourise due to their synthetic origin. Government legislation is becoming more and more stringent, especially in the more developed countries, regarding the removal of dyes from industrial effluents. Concern arises, as several dyes are made from known carcinogens such as benzidine and other aromatic compounds. Most currently existing processes to treat dye wastewater are ineffective and not economical. Therefore, the development of processes based on laccases seems an attractive solution due to their potential in degrading dyes of diverse chemical structure, including synthetic dyes currently employed in the industry. The use of laccase in the textile industry is growing very fast, since besides to decolourise textile effluents as commented above, laccase is used to bleach textiles and even to synthetise dyes. Related to textile bleaching, in 1996 Novozyme (Novo Nordisk, Denmark) launched a new industrial application of laccase enzyme in denim finishing: DeniLite®, the first industrial laccase and the first bleaching enzyme acting with the help of a mediator molecule. Also, in 2001 the company Zytex (Zytex Pvt. Ltd., Mumbai, India) developed a formulation based on LMS capable of degrading indigo in a very specific way. The trade name of the product is Zylite.
Laccase is used in commercial textile applications to improve the whiteness in conventional bleaching of cotton and recently biostoning. Potential benefits of the application include chemicals, energy, and water saving. Cellulases were used to partially replace the load of pumice stones and laccases could bleach indigo-dyed denim fabrics to lighter shades. Laccase also can be used in situ to convert dye precursors for better, more efficient fabric dyeing. In the last few years, various patents reported on coloration achieved with laccase. Laccases find potential applications for cleansing, such as cloth- and dishwashing. Laccase may be included in a cleansing formulation to eliminate the odor on fabrics, including cloth, sofa surface, and curtain, or in a detergent to eliminate the odor generated during cloth washing.
Bl´anquez et al. [154] used T. versicolour in the form of pellets to treat a black liquors discharge for detoxifying and reducing the colour, aromatic compounds, and chemical oxygen demand (COD). They found that colour and aromatic compounds were reduced up to 70–80% and COD was reduced up to 60%. They concluded that T. versicolour is able to produce laccase. T. versicolour completely decolorizes the Amaranth, Tropaeolin O, Reactive Blue 15, Congo Red, and Reactive Black 5 with no dye sorption while it partially decolorizes Brilliant Red 3G-P, Brilliant Yellow 3B-A and Remazol Brilliant Blue R with some dye sorption. They found that after decolourization, toxicity of few dyes remained the same while some became nontoxic [155]. Laccase are also used in dechlorination process. Xylidine is a laccase inducer which increases dechlorination activity due to which dissolved oxygen concentration is reduced. Romero et al. [156] found that bacteria S. maltophilia decolorizes some synthetic dyes (methylene blue, methyl green, toluidine blue, Congo red, methyl orange, and pink) as well as the industrial effluent.
* Nanobiotechnology
Since laccases are able to catalyse electron transfer reactions without additional cofactors, their use has also been studied in biosensors or bioreportersto detect various phenolic compounds, oxygen or azides. Moreover, biosensors for detection of morphine and codeine, catecholamines, plant flavonoids, for determination of glucose, aromatic amines and phenolic compoundsand also for electroimmunoassay have been developed. Nanotechnology contributes to the development of smaller and more efficient biosensors through controlled deposition and specific adsorption of biomolecules on different types of surfaces, achieving micro and nanometer order. Hammond and Whitesides (1995)have introduced a method to pattern ultrathin ionic multilayer films withmicron-sized features onto surfaces building a patterned alkanethiol monolayer with ionic functionality onto a gold surface[157]. Typical molecules used in this process are Polylectrolytes such as PSS: poly(styrene sulfonate); PAA: Poly(acrylic acid), PAH: poly(allylamine hydrochlorid), PDADMAC: poly(diallyldimethylammonium chloride).
Chen et al. (1998)showed a biotechnological application of such micropatterned surfaces: the production of islands of micrometer size of extracellular matrix, where the pattern of these islands could determine the position and distribution of bovine and endothelial cells [158]. The control of the nature and the density of the groups (e.g. alkys, amides, alcohols) of a surface built with assembled monolayers has been used succesfully to investigate the non-specific adsorption of proteins. Regarding laccases, the immobilisation has an important influence on the biosensor sensitivity.
More recently, Cabrita et al. (2005)have immobilised laccase from Coriolus versicolor on N-Hydroxysuccinimide-terminated self-assembled monolayers on gold [159]. This procedure could be useful for the further development of biosensors. In addition, an enzyme electrode based on the co-immobilisation of an osmium redox polymer and a laccase from T. versicolor on glassy carbon electrodes has been applied to ultrasensitive amperometric detection of the catecholamine neurotransmitters dopamine, epinephrine and norepinephrine, attaining nanomolar detection limits. Laccase can also be immobilized on the cathode of biofuel cells that could provide power, for example, for small transmitter systems. Biofuel cells are extremely attractive from an environmental point of view because electrical energy is generated without combusting fuel, thus, providing a cleaner source of energy.
Fig. 2a shows different functionalised flat surfaces built with polymers and self-assembled monolayers (SAMs) that can be used to adsorb and immobilise proteins or other biomolecules. The layer-by-layer technique (LbL) can be used to build macromolecular structures down to nanometer control leading to surfaces of well-defined thickness (Fig. 2a). Recently, flat polyelectrolyte multilayers built by alternating adsorption of oppositely charged polyelectrolytes have been used to recrystallise bacterial proteins making the building of artificial cell walls possible. The LbL technique has also been used to build hollow polyelectrolyte capsules after core removal. Further application of the sequential adsorption of oppositely charged polyelectrolytes onto enzyme crystal templates would permit their encapsulation. Caruso et al. (2000)showed that the encapsulated enzyme could retain 100% of its activity after incubation for 100 min with protease [160]. The permeability properties of the wall capsule are important for the proper function of the encapsulated enzyme.
Antipov et al. (2002)investigated the permeability properties of hollow polyelectrolyte multilayer capsules as a function of pH and salt concentration [161]. It was shown that the capsule wall was closed to a pH value of 8 and higher, but at pH values lower than 6 the macromolecules permeate into the capsule interior. In this way, the authors showed how to open and close the capsule wall in a reversible way. This mechanism together with the LbL encapsulation technique permits the development of microreactors Also, colloidal particles covered with polyelectrolytes and phospholipids have been used to host and activate rubella virus. This type of system is shown in Fig. 2b.

Figure5 :
(a)2D supramacromolecular structures that can be used to immobilise biomolecules. Several structures are suitable: polylectrolyte multilayer, micropatterning and self-assembled monolayers (SAMs).
(b)3D supramacromolecular structures that can be used to build microreactors and immobilise biomolecules. In the first case, hollow polelectrolyte shells can host proteins inside, permitting the diffusion of molecules through the shell wall. A colloidal particle covered by polyelectrolytes (and phospholipids) can host proteins and/or other types of functional molecules.
Laccase covalently conjugated to a bio-binding molecule can be used as a reporter for immunochemical (ELISA, Western blotting), histochemical, cytochemical, or nucleic acid-detection assays. The bioreporter applications are of interest for the high-sensitivity diagnostic field. Fuel cells are very attractive energy sources, particularly at micro-, mini-, portable-, or mobile-scale, that potentially have higher energy conversion/usage efficiency and lower pollution effect than any of the existing/emerging energy sources.
For example, a bio-implantable electrochemical cell system for active implantable medical devices is described by Choi [162]. In one embodiment, the fuel cell includes an electrode structure consisting of immobilized anode and cathode enzymes deposited on nanostructured high-surface-area metal nanowires or carbon nanotube electrodes (Fig. 6). The anode enzyme comprises immobilized glucose oxidase and the cathode enzyme comprises immobilized laccase. Glucose is oxidized at the surface of the anode and oxygen is reduced at the surface of the cathode. The coupled glucose oxidation/oxygen reduction reactions provide a self-generating current source. In another embodiment, the nanowires or carbon nanotubes, along with the adjacent surface anode and cathode electrodes, are coated with immobilized glucose oxidase and immobilized laccase containing biocolloidal substrates, respectively. This results in the precise construction of enzyme architecture with control at the molecular level, while increasing the reactive surface area and corresponding output power by at least two orders of magnitude. Laccase may be applied as a biocatalyst for the electrode reactions. Laccase-based miniature biological fuel cell is of particular interest for many medical applications calling for a power source implanted in a human body.
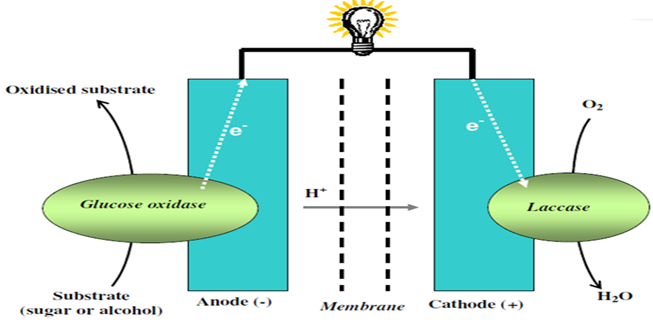
Figure 6 : Schematic representation of a biofuel cell involving glucose oxidase and laccase enzymes.
* Bioremediation and Biodegradation
Laccases have many possible applications in bioremediation. Laccase may be applied to degrade plastic waste having olefin units. Likely, an oxidation of the olefin units by the LMS, could initiate a radical chain reaction, leading to the disintegration of the plastic. Also this LMS can be used to degrade polyurethanes. LMS facilitated the degradation of phenolic compounds (environmental hormones) from biphenol and alkylphenol derivatives and also the decomposition of fluorescent brighteners [165, 166].
Laccase may also be used to eliminate odor emitted from places such as garbage disposal sites, livestock farms, or pulp mills. Also, they could be used for decolorizing dye house effluents that are hardly decolorized by conventional sewage treatment plants. In addition to dye house effluents, laccases can decolorize waste waters from olive oil mills and pulp mills by removing colored phenolic compounds. Another potential environmental application for laccases is the bioremediation of contaminated soils, as laccases and LMS are able to oxidize toxic organic pollutants, such as various xenobiotics, polycyclic aromatic hydrocarbons (PAHs), chlorophenols, and other contaminants.The catalytic properties of laccases can be used to degrade such compounds. Phenolic compounds are present in wastes from several industrial processes, as coal conversion, petroleum refining, production of organic chemicals and olive oil production among others. Immobilized laccase was found to be useful to remove phenolic and chlorinated phenolic pollutants. Laccase was found to be responsible for the transformation of 2,4,6- trichlorophenol to 2,6-dichloro-1,4-hydroquinol and 2,6-dichloro-1,4-benzoquinone. LMSs have been also used to oxidize alkenes, carbazole, N-ethylcarbazole, fluorene, and dibenzothiophene. Isoxaflutole is an herbicide activated in soils and plants to its diketonitrile derivative, the active form of the herbicide: laccases are able to convert the diketonitrile into the acid. Laccase can be also used to reduce the concentration of synthetic heterocyclic compound such as halogenated organic pesticides in the soil. LMS has been extensively study in the oxidation of recalcitrant PAHs, main components of several ship spills. In this sense, LMS is being included in several enzymatic bioremediation programs. Cerrena unicolosr produces laccase in the low nitrogen medium which has thecapability of reducing lignin content from sugarcane bagasseup to 36% within 24 h at 30?C [163].
* Organic synthesis
Recently, increasing interest has been focused on the application of laccase as a new biocatalyst in organic synthesis [53,164]. Laccase provided an environmentally benign process of polymer production in air without the use of H2O2. Laccase-catalyzed cross-linking reaction of new urushiol analogues for the preparation of “artificial urushi” polymeric films (Japanese traditional coating) was demonstrated. It is also mentioned that laccase induced radical polymerization of acrylamide with or without mediator. Laccases are also known to polymerize various amino and phenolic compounds. Recently, to improve the production of fuel ethanol from renewable raw materials, laccase from T. versicolor was expressed in S. cerevisiae to increase its resistance to (phenolic) fermentation inhibitors in lignocellulose hydrolyzates. The preparation of crosslinked enzyme aggregrates with aldehydes and amines had improved stability and was used in starch oxidation. Also, LMS was used for the determination of monoclonal antibody of azelaic acid from oleic acid. The enzymatic preparation of polymeric polyphenols by the action of laccases has been investigated extensively in the past decades as a viable and non-toxic alternative to the usual formaldehyde-based chemical production of these compounds. Laccase can also be used to synthesize various functional organic compounds including polymers with specific mechanical/electrical/optical properties, textile dyes, cosmetic pigments, flavor agents, and pesticides.
* Laccase Function in Insects
Laccase has been found in the cuticles of many insect species [167, 168] and is involved in cuticle sclerotization. Laccases oxidizes catechols in the cuticle to their corresponding quinones, which catalyzes protein cross-linking reactions. In several holometabolous insects, laccase has been identified as the principal enzyme associated with cuticular hardening. The insect laccase is a long amino-terminal sequence characterized by a unique domain consisting of several conserved cysteine, aromatic, and charged residues. In recent years, cloning of insect laccase genes has been performed [27] and two main forms have been found: laccase-1 and laccase-2 [27]. Laccase-1 was found to be expressed in the midgut, Malpighian tubules and fat body as well as the epidermis of the tobacco hornworm, Manduca sexta, and may oxidize toxic compounds ingested by the insect. On the other hand, laccase-2 was involved in cuticle tanning of the red flour beetle, Tribolium castaneum [27]. Recently, a laccase in the salivary glands of N. cincticeps was identified, which is secreted in watery saliva, by using biochemical and histochemical approaches. A possible function of salivary laccase (diphenoloxidase) is in the enhancement of the oxidative gelling occurring in the stylet sheath by a quinone-tanning reaction and rapid oxidization of potentially toxic monolignols to nontoxic polymers during feeding.
* Pharmaceutical Sector
Many products generated by laccases are antimicrobial, detoxifying, or active personal-care agents. Due to their specificity and bio-based nature, potential applications of laccases in the field are attracting active research efforts. Laccase can be used in the synthesis of complex medical compounds as anesthetics, anti-inflammatory, antibiotics, sedatives, etc., including triazolo(benzo)cycloalkyl thiadiazines, vinblastine, mitomycin, penicillin X dimer, cephalosporins, and dimerized vindoline [169].
One potential application is laccase-based in situ generation of iodine, a reagent widely used as disinfectant. Also, laccase has been reported to possess significant HIV-1 reverse transcriptase inhibitory activity [170]. Another laccase has been shown capable of fighting aceruloplasminemia (a medical condition of lacking ceruloplasmin, a multi-Cu serum oxidase whose ferroxidase activity regulates iron homeostasis) [171]. Some years ago, a new enzymatic method based on laccase was developed to distinguish simultaneously morphine and codeine in drug samples injected into a flow detection system [172].
A novel application field for laccases is in cosmetics. For example, laccase-based hair dyes could be less irritant and easier to handle than current hair dyes. More recently, cosmetic and dermatological preparations containing proteins for skin lightening have also been developed. Laccases may find use as deodorants for personal-hygiene products, including toothpaste, mouthwash, detergent, soap, and diapers. Protein engineered laccase may be used to reduce allergenicity.
Table 7 : Some prices of commercially available laccases [151]
|
Source |
Quantity (Units) |
Price |
|
Agaricus bisporus |
10.000
100.000 |
305.00 (US$)
1.560.00 (US$) |
|
Coriolus versicolor |
10.000
100.000 |
250.00 (US$)
1.290.00 (US$) |
|
Pleurotus ostreatus |
10.000 (concentrate) 10.000 (purified) 100.000 (concentrate) 100.000 (purified) |
150.00 (US$)
400.00 (US$)
650.00 (US$)
1,600.00 (US$) |
|
USBiological (www.usbio.net/) From heterologus expression of Trametes versicolor laccase in Saccharomyces cerevisiae |
100 (purified) |
169.00 (US$) |
|
Sigma-Aldrich From Rhus vernicfiera From Agaricus bisporus (≥1.5U/mg) From Coriolus versicolor (≥1U/mg) |
10,000 1 g
5 g 1 g 10 g |
72.30 (US$) 30.50 (US$)
120.90 (US$) 44.00 (US$) 358.20 (US$) |
|
Jena BioScience From Trametes versicolor, Coprinus cinereus and Pycnoporus cinnabarinus |
100U 1000U |
15.00 (EUR) 75.00 (EUR) |
FUTURE TRENDS AND PERSPECTIVES
This review summarizes the available recent important about the properties, heterologous production, and molecular cloning of fungal laccases and possible industrial and biotechnological use. Laccases are promising enzymes to replace the conventional chemical processes of several industries such as the pulp and paper, textile, pharmaceutical, and nanobiotechnology. However, one of the limitations to the large-scale application of laccases is the lack of capacity to produce large volumes of highly active enzyme at an affordable cost (Table 7 - The methodology and expression of laccase activity (Units) are different among the companies). The use of inexpensive sources for laccase production is being explored in recent times.
Thus, efforts have to be made in order to achieve cheap overproduction of laccase in heterologous hosts, and also their modification by chemical means or protein engineering, to obtain more robust, active and less expensive enzymes. Another additional problem is the cost and toxicity of redox mediators. Further investigations should consider different and less polluting mediators such as the natural mediators produced by laccase in a bio-environment during lignin degradation. The current development in laccase catalysis research and the design of mediators along with the research on its heterologous expression opens a wide spectrum of possible applications in the near future. Moreover, laccase can also offer a simple and convenient alternative to using peroxidases with H2O2, because laccases are available on an economically feasible scale.
On the other hand, the development of an effective system for laccase immobilisation also deserves great attention. Immobilisation could be achieved by chemical modification of the substrates. Hence, micropatterning, SAMs and LbL techniques can be used to functionalise flat and curved surfaces in order to have specific adsorption. Laccase encapsulation with polyelectrolytes will be used as a microreactor for catalytic reactions by changing the permeability properties of the capsule wall.
Since the general goal is to obtain stable catalysts with long life times and low cost, the combination of these techniques will enhance :
· the adsorption of laccase on a suitable substrate,
· the lifetime of the laccase activity and
· reutilisation of the substrate/laccase product.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
Laccases are ubiquitous enzymes produced by many living species. They are capable of degrading lignin and are present abundantly in many white-rot fungi. They decolorize and detoxify the industrial effluents and help in wastewater treatment. They act on both phenolic and nonphenolic lignin-related compounds as well as highly recalcitrant environmental pollutants which help researchers to put them in various biotechnological applications. They can be effectively used in paper and pulp industries, textile industries, xenobiotic degradation, and bioremediation and act as biosensor. Laccase has been applied to nanobiotechnology which is an increasing research field and catalyzes electron transfer reactions without additional cofactors. Recently several techniques have been developed for the immobilization of biomolecule such as micropatterning, self-assembled monolayer, and layer-bylayer technique which immobilize laccase and preserve their enzymatic activity.
The introduction of the laccase-mediator system provides a biological alternative to traditional chlorine bleaching processes. laccase or laccase mediator systems offer advantages for the catalysis of the modification of lignin without affecting the properties of the cellulosic material and by avoiding the use of corrosive and toxic conventional methods. Likewise, Laccase catalysis offers the possibility to produce a wide range of new compounds with interesting characteristics, which can be applied in the medical and industrial fields.
One of the limitations to the large-scale application of the enzyme is the lack of capacity to produce large volumes of highly active enzyme. These problems can be solved with the use of recombinant organisms or screening for natural hypersecretory strains. Environmental factors influence the ability of fungi to produce high titres of laccase, and different strains react differently to these conditions. One should thus select a strain capable of producing high concentrations of a suitable enzyme and then optimise conditions for laccase production by the selected organism. Hence laccase is receiving much attention of researchers around the globe.
The major bottlenecks of fungal laccases for large-scale applications are regarded to be their poor stability during long-term use and their low pH optima. The treatment of textile wastewaters, for example, requires enzymes with high stability and activity under alkaline conditions. Nevertheless, the biodiversity of different environments as well as laboratory evolution may provide new laccases - more powerful and specific - to be used in future applications.
REFERENCES
[1] P.R.Williamson, (1994). “Biochemical and molecular characterization of the diphenol oxidase of cryptococcus neoformans : identification as a laccase,” Journal of Bacteriology, vol. 176, no. 3, pp. 656–664.
[2] P.Baldrian, (2006). “Fungal laccases-occurrence and properties,” FEMS Microbiology Reviews, vol. 30, no. 2, pp. 215–242.
[3] N.Dur´an, M.A.Rosa, A.D’Annibale, L.Gianfreda, (2002). “Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review,” Enzyme and Microbial Technology, vol. 31, no. 7, pp. 907–931.
[4] C.F.Thurston, (1994). The structure and function of fungal laccases. Microbiology, 140: 19-26.
[5] F.Xu, (1996). Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry, 35:7608.7614.
[6] F.Xu, (2005). Industrial Biotechnology, 1: 38-50.
[7] A.E.Gardiol, R.J.Hernandez, B.R.Harte, (1998). Device for detecting oxygen with oxidase. United States Patent 5,804,401.
[8] A.Beloqui, M.Pita, J.Polaina, A.Martinez-Arias, O.V.Golyshina, M.Zumarraga, M.M.Yakimov, H.Garcia-Arellano, M.Alcalde, V.M.Fernandez, K.Elborough, J.M. Andreu, A.Ballesteros, F.J.Plou, K.N.Timmis, M.Ferrer, P.N.Golyshin, (2006). Novel polyphenol oxidase mined from a metagenome expression library of bovine rumen-Biochemical properties, structural analysis, and phylogenetic relationships. J Biol Chem, 281: 22933-22942.
[9] S.R.Couto, J.L.Toca Herrera, (2006). “Industrial and biotechnological applications of laccases: a review,” Biotechnology Advances, vol. 24, no. 5, pp. 500–513
[10] J.Faccelo, O.Cruz, (2008). Banana skin: a novel material for a low-cost production of laccase, M.S. thesis, Universitat Rovira I Virgili.
[11] P.Bajpai, (1999). Application of enzymes in the pulp and paper industry. Biotechnol Prog, 15: 147–157.
[12] R.Bourbonnais, M.G.Paice, I.D.Reid, P.Lanthier, M.Yaguchi, (1995). Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2-azinobis(3-ethylbenzthiazoline-6-sulphonate) in kraft lignin depolymerisation. Appl Environ Microbiol, 61: 1876–1880.
[13] A.Breen, F.L.Singleton, (1999). Fungi in lignocellulose breakdown an biopulping, Curr Opin Biotechnol, 10: 252–258.
[14] E.Abadulla, T.Tzanov, S.Costa, K.H.Robra, P.A.Cavaco, G.M.Guebitz, (2000). Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol, 66: 3357–3362.
[15] A.Kandelbauer, O.Maute, R.W.Kessler, A.Erlacher, G.M.Guebitz, (2004). Study of dye decolourization in an immobilized laccase enzyme-reactor using online spectroscopy. Wiley Periodicals, Inc. 552-563.
[16] S.B.Pointing, L.L.P.Vrijmoed, (2000). Decolorization of azo and triphenylmethane dyes by Pycnoporus sanguineus producing laccase as the sole phenoloxidase. World J Microbiol Biotechnol, 16: 317– 318.
[17] P.Reyes, M.A.Pickard, R.Vazquez-Duhalt, (1999). Hydroxybenzotriazole increases the range of textile dyes decolorized by immobilized laccase. Biotechnology Letters, 21: 875–880.
[18] M.D.Cameron, S.Timofeevski, S.D.Aust, (2000). Enzymology of Phanerochaete chrysoporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl Microbiol Biotechnol, 54: 751–758.
[19] J.Karam, J.A.Nicell, (1997). Potential applications of enzymes in waste treatment, J Chem Tech Biotechnol, 69: 141–153.
[20] C.Mougin, A.Kollmann, C.Jolivalt, (2002). Enhanced production of laccase in the fungus Trametes versicolor by the addition of xenobiotics. Biotechnology Letters, 24: 139–142.
[21] N.Durán, E.Esposito, (2000). Potential application of oxidative enzymes and phenoloxidases like compounds in wastewater and soil treatment: a review. Appl Catal B Environ, 28: 83–99.
[22] C.Raghukumar, (2000). Fungi from marine habitats: an application in bioremediation. Mycol Res, 104: 1222–1226.
[23] B.W.Bogan, R.T.Lamar, (1996). Polycyclic aromatic hydrocarbondegradation capabilities of Phanerochaete leavis HHB-1625 and its extracellular ligninolytic enzymes. Appl Environ Microbiol, 62: 1597–1603.
[24] A.Majcherczyk, C.Johannes, A.Hutterman, (1998). Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microbiol Tech, 22: 335–341.
[25] M.A.Pickard, R.Roman, R.Tinoco, R.Vazquez-Duhalt, (1999). Polycyclic aromatic hydrocarbon oxidation by white rot fungi, and oxidation by Coriolopsis gallica UAMH 8260 laccase. Appl Environ Microbiol, 65: 3805–3809.
[26] R.Rama, C.Mougin, F.D.Boyer, A.Kollmann, C.Malosse, J.C.Sigoillot, (1998). Biotransformation of benzo[a]pyrene in bench scale reactor using laccase of Pycnoporus cinnabarinus. Biotechnology Letters, 20(12): 1101–1104.
[27] Y.Arakane, S.Muthukrishnan, R.W.Beeman, M.R.Kanost, K.J.Kramer, (2005). “Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning,” Proceedings of The National Academy of Sciences of the United States of America, vol. 102, no. 32, pp. 11337–11342.
[28] S.Riva, (2006). TRENDS Biotechnol, 24: 219-226.
[29] W.G.Levine, (1965). Laccase: a review, In: The biochemistry of copper (Academic Press Inc., New York), pp. 371-385.
[30] R.Bligny, R.Douce, (1983). Excretion of laccase by sycamore (Acer pseudoplatanus L.) cells. Purification and properties of the enzyme. Biochem. J, 209:489.496.
[31] P.Ranocha, G.McDougall, S.Hawkins, R.Sterjiades, G.Borderies, D.Stewart, M.Cabanes-Macheteau, A.M.Boudet, D.Goffner, (1999). Biochemical characterization, molecular cloning and expression of laccases a divergent gene family - in poplar. Eur. J. Biochem, 259:485.495.
[32] A.De Marco, K.A.Roubelakis-Angelakis, (1997). Laccase activity could contribute to cell-wall reconstitution in regenerating protoplasts. Phytochemistry, 46:421.425.
[33] E.Lehman, E.Harel, A.M.Mayer, (1974). Copper content and other characteristics of purified peach laccase. Phytochemistry, 13:1713.1717.
[34] D.M.O’Malley, R.Whetten, W.Bao, C.L.Chen, R.R.Sederoff, (1993). The role of laccase in lignification. Plant J., 4:751.757.
[35] Y.Sato, B.Wuli, R.Sederoff, R.Whetten, (2001). Journal of Plant Research, 114, 147.
[36] W.D.Wosilait, A.Nason, A.J.Terrell, (1954).Journal of Biological Chemistry, 206,271.
[37] R.P.Gregory and D.S.Bendall, (1966). Biochemical Journal, 101, 569.
[38] J.F.D.Dean and K.E.L.Eriksson, (1994). Holzforschung 48 Supplement, 21.
[39] W.Bao, D.M.O’Malley, R.Whetten, R.R.Sederoff, (1993). A laccase associated with lignification in Loblolly pine xylem. Science, 260:672.674.
[40] U.Dwivedi, P.Singh, V.Pandey, A.Kumar, (2011).J Mol Catal B- Enzym, 68: 117-128.
[41] L.Gianfreda, F.Xu, J.M.Bollag, (1999). “Laccases: a useful group of oxidoreductive enzymes,” Bioremediation Journal, vol. 3, no. 1, pp. 1–25.
[42] G.D.Thakker, C.S.Evans, K.K.Rao, (1992). Applied Microbiol Biotechnology, 37,321.
[43] C.Eggert, U.Temp, J.F.D.Dean, K.E.L.Eriksson, (1996). “A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase,” FEBS Letters, vol. 391, no. 1-2, pp. 144–148.
[44] C.Galhaup, H.Wagner, B.Hinterstoisser, D.Haltrich, (2002). Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enz. Microb. Technol., 30:529.536.
[45] S.Rodríguez Couto, D.Moldes, A.Liébanas, A.Sanromán, (2003). Biochem Eng J,15: 21-26.
[46] J.Dong, Y.Zhang, R.Zhang, W.Huang, Y.Zhang, (2005). J Basic Microb,45:190–198.
[47] G.Palmieri, P.Giardina, C.Bianco, B.Fontanella, G.Sannia, (2000). Applied and Environmental Microbiology, 66, 920.
[48] D.S.Yaver, R.M.Berka, S.H.Brown, F.Xu, (2001). The presymposium on recent advances in lignin biodegradation and biosynthesis, Vikki Biocentre, University of Helsinki, Finland, pp.40.
[49] D.Schlosser, R.Grey, W.Fritsche, (1997). Applied Microbial Biotechnology, 47, 412.
[50] S.C.Froehner, K.E.L.Eriksson, (1974). Journal of Biotechnology, 120, 458.
[51] N.Bar-Nun, A.Tal-Lev, E.Harel, A.M.Mayer, (1988). Repression of laccase formation in Botrytis cinerea and its possible relation to phytopathogenicity. Phytochemistry, 27:2505.2509.
[52] D.Rigling, N.K.van Alfen, (1991). Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J. Bacteriol, 173:8000.8003.
[53] A.M.Mayer, R.C.Staples, (2002). Laccase: new functions for an old enzyme. Phytochemistry, 60:551.565.
[54] P.R.Williamson, (1994). Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J. Bacteriol., 176:656.664.
[55] A.J.Clutterbuck, (1972). Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J. Gen. Microbiol., 70:423.435.
[56] R.Aramayo, W.E.Timberlake, (1990). Sequence and molecular structure of the Aspergillus nidulans yA (laccase I) gene. Nucleic Acids Res., 18:3415.3415.
[57] J.J.Worral, I.Chet, A.Hüttermann, (1986). Association of rhizomorph formation with laccase activity in Armillaria spp. J. Gen. Microbiol., 132:2527.2533.
[58] G.F.Leatham, M.A.Stahmann, (1981). Studies on the laccase of Lentinus edodes: specificity, localization and association with the development of fruiting bodies. J. Gen. Microbiol., 125:147.157.
[59] S.Chen, W.Ge, J.A.Buswell, (2004). Biochemical and molecular characterization of a laccase from the edible straw mushroom, Volvariella volvacea. Eur. J. Biochem., 271:318.328.
[60] J.M.Henson, M.J.Butler A.W.Day, (1999). Annual Review of Phytopathology, 37, 447.
[61] J.Zhao, H.S.Kwan, (1999). Applied and Environmental Microbiology, 65, 4908.
[62] S.D.Salas, J.E.Bennett, K.J.Kwon-Chung, J.R.Perfect, P.R.Williamson, (1996). Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med, 184: 377–386.
[63] P.R.Williamson, K.Wakamatsu, S.Ito, (1998). Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol, 180: 1570–1572
[64] H.F.Tsai, M.H.Wheeler, Y.C.Chang, K.J.Kwon-Chung, (1999). A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol, 181: 6469–6477.
[65] A.Givaudan, A.Effose, D.Faure, P.Potier, M.L.Bouillant, R.Bally, (1993). Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol. Lett., 108:205.210.
[66] D.Faure, M.L.Bouillant, R.Bally, (1994). Isolation of Azospirillum lipoferum 4T Tn5 mutants affected in melanization and laccase activity. Appl. Environ. Microbiol., 60:3413.3415.
[67] C.Held, A.Kandelbauer, M.Schroeder, A.Cavaco-Paulo G.Guebitz, (2005). Environ Chem Lett, 3: 74–77.
[68] P.Sharma, R.Goel N.Capalash, (2007). World J Microbiol Biotechnol, 23:823–832.
[69] K.Endo, K.Hosono, T.Beppu, K.Ueda, (2002). A novel extracytoplasmatic phenol oxidase of Streptomyces: its possible involvement in the onset of morphogenesis. Microbiology, 148: 1767–1776.
[70] F.Solano, E.Garcia, E.Perez De Egea, A.Sanchez-Amat, (1997). Isolation and characterization of strain MMB-1 (CECT 4803), a novel melanogenic marine bacterium. Appl. Environ. Microbiol. 63:3499.3506.
[71] A.Sanchez-Amat, P.Lucas-Elio, E.Fernandez, J.C.Garcia-Borron, F.Solano, (2001). Molecular cloning and functional characterization of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. Biochim. Biophys. Acta, 1547:104.116.
[72] L.O.Martins, C.M.Soares, M.M.Pereira, M.Teixeira, T.Costa, G.H.Jones, A.O. Henriques, (2002). Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem., 277:18849.18859.
[73] M.Berrocal, J.Rodríguez, A.S.Ball, M.I.Pérez-Leblic, M.E.Arias, (1997). Solubilisation and mineralisation of [14C]lignocellulose from wheat straw by Streptomyces cyaneus CECT 3335 during growth in solid-state fermentation. Appl Microbiol Biotechnol, 48: 379–384.
[74] M.Berrocal, A.S.Ball, S.Huerta, J.M.Barrasa, M.Hernández, M.I.Pérez-Leblic, M.E.Arias, (2000). Biological upgrading of wheat straw through solid-state fermentation with Streptomyces cyaneus. Appl Microbiol Biotechnol, 54: 764–771.
[75] P.Durão, I.Bento, A.Fernandes, E.Melo, P.Lindley, L.Martins, (2006). J Biol Inorg Chem, 11: 514-526.
[76] M.Sugumaran, L.Giglio, H.Kundzicz, S.Saul, V.Semensi, (1992). Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch. Insect Biochem. Physiol., 19:271.283.
[77] N.T.Dittmer, R.J.Suderman, H.Jiang, Y.C.Zhu, M.J.Gorman, K.J.Kramer, M.R.Kanost, (2004). Characterization of cDNAs encoding putative laccaselike multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol., 34:29.41.
[78] H.Claus, (2004). Micron, 35: 93–96[79] A.Kunamneni, F.Plou, A.Ballesteros, M.Alcalde, (2008). Recent Patents on Biotechnology 2: 10-24.
[80] M.Heinzkill, L.Bech, T.Halkier, P.Schneider, T.Anke, (1998). Applied and Environmental Microbiology, 64, 1601.
[81] T.Suzuki, K.Endo, M.Ito, H.Tsujibo, K.Miyamoto, Y.Inamori, (2003). A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci. Biotechnol. Biochem.,67:2167.2175.
[82] V.Ducros, A.M.Brzozowski, K.S.Wilson, S.H.Brown P.Ostergaard, P.Schneider, D.S.Yaver, A.H.Pedersen, G.J.Davies, (1998). Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 angstrom resolution. Nat Struct Biol, 5: 310–316.
[83] K.Piontek, M.Antorini, T.Choinowski, (2002). Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem, 277: 37663-37669.
[84] M.Antorini, I.Herpoel-Gimbert, T.Choinowski, J.C.Sigoillot, M.Asther, K.Winterhalter, K.Piontek, (2002). Purification, crystallisation and X-ray diffraction study of fully functional laccases from two ligninolytic fungi. Biochim Biophys Acta, 1594: 109–114.
[85] N.Hakulinen, L.L.Kiiskinen, K.Kruus, M.Saloheimo, A.Paananen, A.Koivula, J.Rouvinen, (2002). Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol, 9: 601–605.
[86] S.Garavaglia, M.T.Cambria, M.Miglio, S.Ragusa, V.Lacobazzi, F.Palmieri, C.D’Ambrosio, A.Scaloni, M.Rizzi, (2004). The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J Mol Biol, 342: 1519-1531.
[87] F.J.Enguita, L.O.Martins, A.O.Henriques, M.A.Carrondo, (2003). Crystal structure of a bacterial endospore coat component – a laccase with enhanced thermostability properties. J Biol Chem, 278: 19416–19425.
[88] F.J.Enguita, D.Marcal, L.O.Martins, R.Grenha, A.O.Henriques, P.F.Lindley, M.A.Carrondo, (2004). Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J Biol Chem, 279: 23472–23476.
[89] S.Riva, (2006). “Laccases: blue enzymes for green chemistry,” Trends in Biotechnology, vol. 24, no. 5, pp. 219–226.
[90] S.Rodríguez Couto, J.Toca-Herrera, (2006). Biotechnology Advances, 24: 500– 513.
[91] V.Madhavi, S.Lele, (2009). Bioresources, 4: 1694-1717.
[92] R.Bourbonnais, M.G.Paice, B.Freiermuth, E.Bodie, S.Borneman, (1997). Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol, 12: 4627-4632.
[93] S.Camarero, D.Ibarra, M.J.Martinez, A.T.Martinez, (2005). Applied and Environmental Microbiology, 71, 1775.
[94] P.Cassland, L.J.Jonsson, (1999). Characterization of a gene encoding Trametes versicolor laccase A and improved heterologous expression in Saccharomyces cerevisiae by decreased cultivation temperature. Appl. Microbiol. Biotechnol., 52:393.400.
[95] B.Valderrama, P.Oliver, A.Medrano-Soto, R.Vazquez-Duhalt, (2003). Evolutionary and structural diversity of fungal laccases. Ant. v. Leeuwenh., 84:289.299.
[96] R.Aramayo, W.E.Timberlake, (1993). The Aspergillus nidulans yA gene is regulated by abaA. EMBO J., 12:2039.2048.
[97] M.Scherer, R.Fischer, (2001). Molecular characterization of a blue-copper laccase, TILA, of Aspergillus nidulans. FEMS Microbiol. Lett., 199:207.213.
[98] A.Schouten, A.L.Van Kan Johannes, F.L.Stefanato, C.A.M.Sibbel-Wagemakers, (2002). EP1167528 A1.
[99] C.Salas, S.Lobos, J.Larrain, L.Salas, D.Cullen, R. Vicuña, (1995). Properties of laccase isoenzymes produced by the basidiomycete Ceriporiopsis subvermispora. Biotechnol. Appl. Biochem., 21: 323.333.[100] E.Karahanian, G.Corsini, S.Lobos, R.Vicuña, (1998). Structure and expression of a laccase gene from the ligninolytic basidiomycete Ceriporiopsis subvermispora. Biochim. Biophys. Acta., 1443:65.74.
[101] D.S.Yaver, M.J.Overjero, F.Xu, B.A.Nelson, K.M.Brown, T.Halkier, S.Bernauer, S.H.Brown, S.Kauppinen, (1999). Molecular characterization of laccase genes from the basidiomycete Coprinus cinereus and heterologous expression of the laccase lcc1. Appl. Environ. Microbiol., 65:4943.4948.
[102] W.A.Edens, T.Q.Goins, D.Dooley, J.M.Henson, (1999). Purification and characterization of a secreted laccase of Gaemannomyces graminis var. tritici. Appl Environ Microbiol., 65: 3071-3074.
[103] B.Dedeyan, A.Klonowska, S.Tagger, T.Tron, G.Iacazio, G.Gil, J.Le Petit, (2000). Biochemical and molecular characterization of a laccase from Marasmius quercophilus. Appl. Environ. Microbiol., 66:925.929.
[104] K.Kruus, L.L.Kiiskinen, M.Ratto, L.Viikari, M.Saloheimo, (2007). US7183090.
[105] R.M.Berka, S.H.Brown, F.Xu, P.Schneider, K.M.Oxenbøll, D.A.Aaslyng, (1997a). Purified Myceliophthora laccases and nucleic acids encoding same. USA Pat. Appl., US5981243.
[106] R.M.Berka, P.Schneider, E.J.Golightly, S.H.Brown, M.Madden, K.M.Brown, T.Halkier, K.Mondorf, F.Xu, (1997b). Characterization of the gene encoding an extracellular laccase of Myceliophtora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl. Environ. Microbiol., 63:3151.3157.
[107] U.A.Germann, G.Müller, P.E.Hunziker, K.Lerch, (1988). Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J. Biol. Chem., 263:885.896.
[108] M.L.Niku-Paavola, E.Karhunen, P.Salola, V.Raunio, (1988). Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem. J., 254:877.884.
[109] P.Giardina, G.Palmieri, A.Scaloni, B.Fontanella, V.Faraco, G.Cennamo, G.Sannia, (1999). Protein and gene structure of a blue laccase from Pleurotus ostreatus. Biochem. J., 341:655.663.
[110] G.Palmieri, P.Giardina, L.Marzullo, B.Desiderio, G.Nitti, R.Cannio, G.Sannia, (1993). Stability and activity of a phenol oxidase from the ligninolytic fungus Pleurotus ostreatus. Appl. Microbiol. Biotechnol., 39:632.636.
[111] P.M.Coll, J.M.Fernandez-Abalos, J.R.Villanueva, R.Santamaria, P.Perez, (1993a). Purification and characterization of a phenoloxidase (laccase) from the lignin-degrading basidiomycete PM1 (CECT 2971). Appl. Environ. Microbiol., 59:2607.2613.
[112] P.M.Coll, C.Tabernero, R.Santamaria, P.Perez, (1993b). Characterization and structural analysis of the laccase I gene from the newly isolated ligninolytic basidiomycete (CECT 2971). Appl. Environ. Microbiol., 59:4129.4135.
[113] J.Fernández-Larrea, U.Stahl, (1996). Isolation and characterization of a laccase gene from Podospora anserina. Mol. Gen. Genet., 252:539.551.
[114] J.A.Wahleithner, F.Xu, K.M.Brown, S.H.Brown, E.J.Golightly, T.Halkier, S.Kauppinen, A.Pederson, P.Schneider, (1996). The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr. Genet., 29:395.403.
[115] C.Galhaup, S.Goller, C.K.Peterbauer, J.Strauss, D.Haltrich, (2002). Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology, 148:2159.2169.
[116] M.Colao, A.M.Garzillo, V.Buonocore, A.Schiesser, M.Ruzzi, (2003). Primary structure and transcription analysis of a laccase-encoding gene from the basidiomycete Trametes trogii. Appl. Microbiol. Biotechnol., 63:153.158.
[117] E.Ong, W.B.Pollock, M.Smith, (1997). Cloning and sequence analysis of two laccase complementary DNAs from the ligninolytic basidiomycete Trametes versicolor. Gene, 196:113.119.
[118] P.Cassland, L.J.Jonsson, (1999). Characterization of a gene encoding Trametes versicolor laccase A and improved heterologous expression in Saccharomyces cerevisiae by decreased cultivation temperature. Appl. Microbiol. Biotechnol., 52:393.400.
[119] D.S.Yaver, E.J.Golightly, (1996). Cloning and characterization of three laccase genes from the white rot basidiomycete Trametes villosa: genomic organization of the laccase gene family. Gene, 181:95.102.
[120] W.Liu, Y.Chao, S.Lii, H.Bao, S.Qian, (2003). Molecular cloning and characterization of a laccase gene from the basidiomycete Fome lignosus and expression in Pichia pastoris. Appl Microbiol Biotechnol., 63: 174-181.
[121] L.F.Larrondo, M.Avila, L.Salas, D.Cullen R.Vicuña, (2003). Heterologous expression of laccase cDNA from Ceriporiopsis subvermispora yields copper-activated apoprotein and complex isoform patterns. Microbiology, 149:1177.1182.
[122] L.L.Kiiskinen, K.Kruus, M.Bailey, E.Ylosmaki M.Siika-aho, M.Saloheimo, (2004). Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterization of the purified enzyme. Microbiol., 150: 3065-3074.
[123] T.Bulter, M.Alcalde, V.Sieber, P.Meinhold, C.Schlachtbauer, F.H.Arnold, (2003). Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl. Environ. Microbiol., 69:987.995.
[124] M.Saloheimo, M.L.Niku-Paavola, (1991). Heterologous production of a ligninolytic enzyme: expression of the Phlebia radiata laccase gene in Trichoderma reesei. Biotechnology, 9:987.990.
[125] D.M.Soden, A.D.W.Dobson, (2002). Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology, 148:4003.4014.
[126] L.Otterbein, E.Record, S.Longhi, M.Asther, S.Moukha, (2000). Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur. J. Biochem., 267:1619.1625.
[127] E.Record, P.J.Punt, M.Chamkha, M.Labat, C.A.M.J.J.van Den Hondel, M.Asther, (2002). Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur. J. Biochem., 269:602.609.
[128] C.Sigoillot, E.Record, V.Belle, J.L.Robert, A.Levasseur, P.J.Punt, C.A.Van Den Hondel, A.Fournel, J.C.Sigoillot, M.Asther, (2004). Natural and recombinant fungal laccases for paper pulp bleaching. Appl. Microbiol. Biotechnol., 64:346.352.
[129] Alves AMCR, E.Record, A.Lomascolo, K.Scholtmeijer, M.Asther, Wessels JGH, Wosten HAB, (2004). Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl Environ Microbiol.,70: 6379-6384.
[130] J.F.Osma, V.Saravia, J.L.T.Herrera, S.R.Couto, (2007). Chemosphere, 67, 1677.
[131] Z.Xing, J.Cheng, Q.Tan, Y.Pan, (2006). World J Microbiol Biotechnol., 22:1215 1221.
[132] X.Lu, S.Ding, (2010). Mycoscience, 51: 68-74.
[133] G.S.Nyanhongo, J.Gomes, G.Gubitz, R.Zvauya, J.S.Read, W.Steiner, (2002). Bioresource Technology, 84, 259.
[134] J.Hess, C.Leitner, C.Galhaup et al., (2002). “Enhanced formation of extracellular laccase activity by the white-rot fungus Trametes multicolor,” Applied Biochemistry and Biotechnology—Part A Enzyme Engineering and Biotechnology, vol. 98-100, pp. 229– 241.
[135] A.P.M.Tavares, M.A.Z.Coelho, M.S.M.Agapito, J.A.P.Coutinho, A.M.R.B.Xavier, (2006). “Optimization and modeling of laccase production by Trametes versicolor in a bioreactor using statistical experimental design,” Applied Biochemistry and Biotechnology, vol. 134, no. 3, pp. 233–248.
[136] S.R.Couto, J.L.Toca-Herrera, (2007). “Laccase production at reactor scale by filamentous fungi,” Biotechnology Advances, vol. 25, no. 6, pp. 558–569.
[137] Laura Mendoza, (2010). Laccase from Galerina sp. HC1.
[138] G.Songulashvili, V.Elisashvili, S.Wasser, E.Nevo, Y.Hadar, (2006). Biotechnol Lett, 28:1425–1429.
[139] N.Hatvani, I.Mécs, (2001). Process Biochem, 37: 491-496.
[140] M.Staji?, L.Persky, D.Friesem, Y.Hadar, S.Wasser, E.Nevo, et al., (2006). Enzyme Microb Technol, 38: 65-73.
[141] M.Lorenzo, D.Moldes, S.Rodríguez Couto, A.Sanromán, (2002). Bioresource Technol, 82: 109-113.
[142] J.Meza, J.Sigoillot, A.Lomascolo, D.Navarro, R.Auria, (2006). J Agric Food Chem, 54: 3852–3858.
[143] E.Rosales, S.Rodruíguez Couto, M.Sanromán, (2005). J Food Eng, 66: 419-423.
[144] E.Rosales, S.Rodruíguez Couto, M.Sanromán, (2007). Enzyme Microb Technol, 40: 1286–1290.
[145] E.Grotewold, G.E.Taccioli, G.O.Aisemberg, N.D.Judewicz, (1998). “Purification of an extracellular fungal laccase,” Mircen Journal of Applied Microbiology and Biotechnology, vol. 4, pp. 357–363.
[146] L.Cordi, R.C.Minussi, R.S.Freire, N.Dur´an, (2007). “Fungal laccase: copper induction, semi-purification, immobilization, phenolic effluent treatment and electrochemicalmeasurement,” African Journal of Biotechnology, vol.6, no. 10, pp. 1255–1259.
[147] S.Khammuang, R.Sarnthima, (2009). “Laccase activity from fresh fruiting bodies of Ganoderma sp. MK05: purification and remazol brilliant blue R decolorization,” Journal of Biological Sciences, vol. 9, no. 1, pp. 83–87.
[148] R.F.Taylor, (1991). Protein immobilization: Fundamental and applications (Marcel Dekker Inc., New York, USA).
[149] P.Peralta-Zamora, C.M.Pereira, E.R.L.Tiburtius et al., (2003). “Decolorization of reactive dyes by immobilized laccase,” Applied Catalysis B, vol. 42, no. 2, pp. 131–144.
[150] E.Selinheimo, K.Kruus, J.Buchert, A.Hopia, K.Autio, (2006). Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J Cereal Sci, 43:152–9.
[151] R.C.Minussi, G.M.Pastore, N.Durán, (2002). Potential applications of laccase in the food industry. Trends Food Sci Technol, 13:205–16.
[152] R.Minussi, M.Rossi, L.Bologna, D.Rotilio, G.Pastore, N.Durán, (2007). J Mol Catal B- Enzym, 45: 102–107.
[153] O.Yesilada, S.Sik, M.Sam, (1998). World J Microbiol Biotechnol, 14: 37-42.
[154] P.Bl´anquez, N.Casas, X.Font et al., (2004). “Mechanism of textile metal dye biotransformation by Trametes versicolor,” Water Research, vol. 38, no. 8, pp. 2166–2172.
[155] J.A.Ramsay, T.Nguyen, (2002). “Decolourization of textile dyes by Trametes versicolour and its effect on dye toxicity,” Biotechnology Letters, vol. 24, pp. 1757–1761.
[156] S.Romero, P.Bl´anquez, G.Caminal et al., (2006). “Different approaches to improving the textile dye degradation capacity of Trametes versicolor,” Biochemical Engineering Journal, vol. 31, no. 1, pp. 42–47.
[157] P.T.Hammond, G.M.Whitesides, (1995). Formation of polymer microstructures by selective deposition of polyion multilayers using patterned selfassembled monolayers as a template. Macromolecules, 28: 7569–71.
[158] C.S.Chen, M.Mrkisch, S.Huang, G.M.Whitesides, D.E.Ingber, (1998). Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog, 14:356–63.
[159] J.F.Cabrita, L.M.Abrantes, A.S.Viana, (2005). N-Hydroxysuccinimide-terminated self-assembled monolayers on gold for biomolecules immobilisation. Electrochim Acta, 50:2117–24.
[160] F.Caruso, D.Trau, H.Möhwald, R.Renneberg, (2000). Enzyme encapsulation in layer-by-layer engineered polymer multilayer capsules. Langmuir, 16:1485–8.
[161] A.Antipov, G.B.Sukhorukov, S.Leporatti, I.L.Radtchenko, E.Donath, H.Möhwald, (2002). Polyelectrolyte nultilayer capsule permeability control. Colloids Surf A Physicochem Eng Asp, 198-200:535–41.
[162] S.H.Choi, (2005). US2005118494 A1.
[163] D.D’Souza-Ticlo, D.Sharma, C.Raghukumar, (2009). “A thermostable metal-tolerant laccase with bioremediation potential from a marine-derived fungus,” Marine Biotechnology, vol. 11, no. 6, pp. 725–737.
[164] O.Milstein, B.Nicklas, A.Hütttermann, (1989). Oxidation of aromatic compounds in organic solvents with laccase from Trametes versicolor. Appl Microbiol Biotechnol 31:70-74.
[165] M.Alcalde, T.Bulter, F.H.Arnold, (2002). Journal of Biomolecular Screening, 7, 537.
[166] A.Majecherczyk, C.Johannes, (2000). Biochimica et Biophysica Acta, 1474, 157.
[167] B.R.Thomas, M.Yonekura, T.D.Morgan, T.H.Czapla, T.L.Hopkins, K.J.Kramer, (1989). “A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta,” Insect Biochemistry, vol. 19, no. 7, pp. 611– 622.
[168] H.I.Yamazaki, (1972). “Cuticular phenoloxidase from the silkworm, bombyx mori: properties, solubilization, and purification,” Insect Biochemistry, vol. 2, no. 8, pp. 431–444.
[169] P.Stahl, L.Kissau, R.Mazitschek, A.Giannis, H.Waldmann, (2002). Natural product derived receptor tyrosin kinase inhibitors: Identification of IGF1R-, Tie-2 and VEGFR3 inhibitors. Angew Chem Int Ed, 41: 1174-1178.
[170] H.X.Wang, T.B.Ng, (2004). A novel laccase with fair thermostability from the edible wild mushroom (Albatrella dispansus). Biochem Biophys Res Commun, 315: 450-454.
[171] Z.L.Harris, S.R.Davis-Kaplan, J.D.Gitlin, J.Kaplan, (2004). A fungal multicopper oxidase restores iron homeostasis in aceruloplasminemia. Blood, 103: 4672-4673.
[172] C.G.Bauer, A.Kuhn, N.Gajovic, O.Skorobogatko, P.J.Holt, N.C.Bruce, A.Makower, C.R.Lowe, F.W.Scheller, (1999). New enzyme sensors for morphine and codeine based on morphine dehydrogenase and laccase.Fresenius J Anal Chem,364:179-183.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









