{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Sumedha Saxena, Chandan Kumar Singh*, MukeshYadav, Alex Laurel Samson.
Department of Pharmacy, Dr. M.C. Saxena College of pharmacy, Lucknow, (U.P.)
*chandansingh.skic@gmail.com
ABSTRACT
This review mainly focus on targeted drug delivery to the colon is extremely desirable for local treatment of inflammatory bowel disease such as ulcerative colitis, crohn's disease, amoebiasis, colonic cancer as well as for systemic delivery of protein and peptide drugs. The colon is a site where local delivery allows topical treatment of inflammatory bowel disease and systemic delivery of drug can take place. Treatment of colon can be made effective if the drug can be targeted directly into the colon because drug release and absorption should not be take in abdomen and the small intestine and bioactive agents should not be degraded and to allow drug release into the colon. Drug targeting to the colonic area is not only associated with the treatment of colonic ailment locally but also delivering drugs such as protein and peptide for their systemic effects which are degraded and bioavailability is very depressed due to the instability in the GI Tract.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2596
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 7 Received On: 22/05/2018; Accepted On: 11/06/2018; Published On: 01/07/2018 How to cite this article: Singh, C.K., Saxena, S., Yadav, M. and Samson, A. 2018. A review on novel approaches for colon targeted drug delivery systems. PharmaTutor. 6, 7 (Jun. 2018), 11-22. DOI:https://doi.org/10.29161/PT.v6.i7.2018.11 |
INTRODUCTION
Colon targeted drug delivery system may be following the concept of control or sustained route for drug administration has received more attention (Vinay et al., 2012). At least 50% of the dosage forms available in the market are oral dosage form because these system have more advantage due to high patient compliances (Akhil et al., 2011) Targeted dreg delivery into the colon is highly desirable for local treatment of a variety of bowel disease. Like crohn's disease, colonic cancer ulcerative Colitis, amebiosis and many others disorders ((Anil and Betty, 2010). Colon targeted drug delivery system is not only the suitable for delivering the drug to the colon for local treatment but it is also a preferable approach for delivery of protein and peptide and enhance their bioavailability (Amidon et al., 2015). Bioavailability of protein and peptide may be achieved if it can be protected form acid and enzymes in the stomach and upper intestine and released and absorbed in the colon and protect it form degradation in stomach (Prasanth et al., 2012). Degradation of such drug in stomach can be prevent by using some polymer either alone or in a combination because polymer effect the rate of release and absorption of drugs and play an important role in formulation of colon targets drug delivery system (H. Rajpurohit et al., 2010). Oral route is the more preferable and convenient route but other routes for colon drug delivery system may be used. Administration of drug through the rectum offers the shortest route for targeting drugs to the colon still reaching the colon proximal part via rectal administration is difficult. Rectal administration is difficult and painful for patient thus patient compliances decreased (Massimo Campieri et al., 1992). Colon contain a variety of bacteria which is responsible for azo reduction and enzymatic cleavage which is responsible for the metabolism of many drugs (Sai Mansa et al., 2015)
Choice of polymer for CTDDS
Delivery of drugs to the colon lead on much interest for local and systemic treatment of variety of colonic disease because of various therapeutic advantage of colon like near neutral and longer transit time. Advantageous delivery of a drug to the colon depend upon protection of the drug from degradation or release in the stomach which can be achieved by using polymer because polymer can influence the rate of release and absorption of drug and have an important role in formulating CTDDS. Both natural and biodegradable polymers are preferred for drug delivery application. The advantage for natural and biodegradable polymers are-
a) In expensive and available in variety of structure.
b) Biodegradable polymers are broken down into biologically acceptable molecules that are metabolized and removed from the body via normal metabolic pathway.
So that the choice of polymer for formulating colon targeted drug delivery system is playing a vital role. (H. Rajpurohit et al., 2010); ( Neha Sharma and harikumar,2013)
Advantage of CTDDS over Conventional Drug
• Colon have less peptidase activity so peptides, insulin oral vaccines, growth hormones can be delivered through this route (Shruti, 2007).
• Colon is ideal site for the delivery of agents to treat the local disease of the colon (Mahesh,2014)
• Required small drug quantities due to locality targeting (Akhil, 2011).
• Reduces dosage frequency Hence lower cost of expensive drugs.
• Possible leading to a reduced incidence of side effects and drug interactions.
• The colon is an attractive site where poorly absorbed drug molecules may have an improved bioavailability.
• Reduce gastric irritation caused by many drugs like NSAIDS.
• Bypass initial first pass metabolism.
• Extended daytime of night time activity.
• Improve patient compliance.
• It has a longer retention time and appears highly responsive to agents that enhance the absorption of poorly absorbed drug (Mahesh et al.,2014); (N.K. Jain, 2001); (M. Halsa,2001); (Rennat,1998;)
Limitation of Colon targeting drug delivery system-
• Multiple manufacturing steps.
• Incomplete release rate.
• Bioavailability of drug may be low due to potentially binding of a drug in a nonspecific way to dietary residue, fecal matter, mucus and intestinal secretion.
• The resident micro flora could also affect colonic performance via metabolic degradation of the drug.
• Non availability of an appropriate dissolution testing method to evaluate the dosage form in-vitro (Akhil et al., 2011); ( Sukhbir et al.,2013).
• Drug should be in the solution form before absorption and there for rate limiting step for poor soluble drugs.
• An important limitation of the PH sensitive coating technique is the uncertainty of the location and environment in which the coating may start to dissolve. Normal in the patient with ulceration colitis (M.K. Ratnaparkhi et al., 2013).
• Limitation of pro-drug approach is that it is not very versatile approach as it's formulation depends upon the functional group available on the drug moiety for chemical linkage.(Gaurav et al., 2010).
|
Targeted site |
Disease |
Drug |
|---|---|---|
|
Topical |
Inflammatory bowel diseases (Crohn's disease, ulcerative colitis), Irritable bowel diseases, Amoebiasis
|
Hydrocortisone, Prednisolone, Sulfasalazine, Mesalazine, Mercaptopurine, Metronidazole, Mebendazole, Tinidazole.
|
|
Systemic |
Oral delivery of peptide, oral delivery of vaccines, to prevent gastric irritation, To prevent first pass metabolism of orally administered drug. |
Typhoid, NSAIDS, Steroids, Insulin |
|
Local |
Pancreatactomy, Colorectal cancer, cystic fibrosis, Chronic pancreatitis |
5- Fluorouracil, digestive enzymes |
TABLE No. 01-Colonic diseases, its sites and active drug components. (Danda and chandan, 2013)
Anatomy of Colon (Prabhakar et al., 2012); ( Anne and Allison, 2010)

Diagram 01- Colon
The digestive system is the collective name used to describe the alimentary canal, its accessory organs and a variety of digestive process. The alimentary canal begin at the mouth, passes through the thorax, abdomen and pelvis and ends at the anus.
The large intestine is about 1.5 meters long, beginning at the caecum in the right iliac fossa and terminating at rectum and anal canal deep in the pelvis. Human large intestine is about 1.5 meter long. the colon is a cylindrical tube which is linked by moist, soft pink lining called mucosa. The colon has four parts which are same structure and function.
Ascending Colon-It is nearly 12.5cm, in length. It extends from the caecum to the hepatic flexure.
Transverse Colon- The ascending colon further continues as the transverse colon, form the hepatic flexure to the splenic future.
Descending Colon- The transverse Colon descends down to form the descending colon. It begins at the splenic mixture and terminates at the beginning of the sigmoid colon.
Sigmoid Colon- The descending colon continue as an S-Shaped Sigmoid colon, terminating at the rectum.
|
Sr. No. |
Large Intestine |
Length (cm) |
|---|---|---|
|
1. |
Cecum |
6-9 |
|
2. |
Ascending Colon |
20-25 |
|
3. |
Descending Colon |
10-15 |
|
4. |
Transverse Colon |
40-45 |
|
5. |
Sigmoid Colon |
35-40 |
|
6. |
Rectum |
12 |
|
7. |
Anal Canal |
3 |
Table No. 02- Length of Various Parts of Large Intestine.
Effect of Colonic bacterial Flora (Sujit K. Chaudhari, 2014)
Beneficial Effects
1) Colonic bacterial flora produce many vitamins include vitamin K and some member of vitamin B complex including folic acid.
2) Colonic bacteria produce short chain fatty acids (SCFA's) which prevent colorectal cancer.
Harmful effects:-
1) The bacterial overgrowth in the ileum can developed diarrhea, Mal absorption syndrome and anemia.
2) The Colonic bacterial flora (some species) can produce ammonia which is not absorbed in hepatic failure and precipitate hepatic encephalopathy
Factor affecting Colon targeted drug delivery system:-
The factors that affect the Colon targeting are categorized in two categories:-
1) Physiological factors
2) Pharmaceutical factors
1) Physiological factors:-
The factor which involve physiologically in drug delivery and responsible for affecting the targeting of drug to the colon are:-
a) Gastric emptying (Danda and chandan, 2013)
Drug delivery to the colon upon oral administration depend mainly on gastric emptying and bowel transit time i.e. upon reaching the colon the transit time of drug depends on the nature and size of the particles. Larger particles have less transit time as compared to smaller particles.
|
Sr. No |
Part of GIT |
Transit Time |
|---|---|---|
|
1 |
Fasted State |
10min- 2hr |
|
2 |
Fed State |
2 hr |
|
3 |
Small intestine |
3-4 hr |
|
4 |
Colon transit |
20-35 hr |
b) pH of colon
The pH of the Gastrointestinal tract is subjected to both inter and intra subject variation. Disease state, Diet and food intake influence the pH of GI Tract. This changes in the pH in different parts of GIT is the basic for the development of Colon targeted drug delivery systems. Coating with different polymers is done to target the drug to the site. (M. Prathap et al., 2014)
|
Part of GIT |
pH |
|
Stomach |
Fasted state 1.5 - 2 Fed state 2.6 |
|
Small Intestine |
6.6 – 7.5 |
|
Colon Ascending Colon Transverse Colon Descending Colon |
6.4 6.6 7.0 |
C) Colonic micro Flora and enzymes (Cynthia and Wendy, 2015); (Danda andChandan, 2013); (M. Prathap et al., 2014)
The Gastrointestinal Contain a variety of microorganism that produces many enzymes need for metabolism. Growth of this micro flora is controlled by the GIT Contentment's and peristaltic movements. The enzyme released by different microorganism E. Coli, Clostridia, Lactobacilli, Eubacteria, Streptococci are responsible for the various metabolic reaction that take place in GIT.
|
Microorganism |
Enzyme |
Metabolic Reaction |
|---|---|---|
|
E.Coli, Bacteroids |
Nitroreductase |
Reduce aromatic and heterocyclic nitro compounds |
|
Clostridea, Lactobacilli |
Hydrogenase |
Reduce carbonyl group and aliphatic double bond. |
|
Clostridia, Eubacteria |
Glycosidase |
Clevage of B-glycosides of alcohols and phenol |
|
Eubacteria Clostridia Streptococci |
Sulfates |
Cleavage of O-Sulphate and Sulfamates |
Table No. 04- Different micro flora, enzymes released and action
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Pharmaceutical Factors-
The Various Pharmaceutical factors are-
a) Drug Candidates-
Drugs which show poor absorption from the stomach or intestine including peptide are most suitable for CDDS. The drug used in the treatment of IBD, ulcerative Colitis, diarrhea and colon cancer are ideal character for local colon delivery. (Mahesh et al., 2014). Due to high retention time of colon, colon causes an increase in the absorption an increase in the absorption of poorly absorbed agents (M. Prathap et al., 2014)
b) Drug Carrier-
The selection of carrier for particular drug candidate depends on the physiochemical nature of the drug as well as the disease for which the system is to be used. The factors such as chemical nature, stability and partition coefficient of drug and the type of absorption enhancers choosen influence the carrier selection, Moreover the choice of drug carrier depends on the functional groups of drug molecule. (Mukesh et al., 2013)
Different approaches used for colon targeting-
1. Primary approaches of colon specific drug delivery-
a) PH Sensitive polymer coated drug delivery system.
b) Delayed (Time Controlled release System) release drug delivery system.
c) Microbial triggered system
d) Pro-drug approach for drug delivery to colon
e) Polysaccharide based delivery system.
2. Newly developed approaches for CDDS-
a) Pressure Controlled drug – delivery system.
b) Pulsatile colon targeted drug delivery.
i) Pulsin Cap system
ii) Port System.
c) CODES technology
d) Osmotic controlled drug delivery (OROS-CT)
e) Multiparticulate system based drug delivery.
f) Azo hydrogels.
g) Probiotic approach.
Primary Approaches of Colon specific drug delivery-
A) pH Sensitive polymer coated drug delivery system.
There is numerous invention approaches have been discovered to develop colon- difference in the pH along the GI tract is one of the approaches to achieve colon targeting. So the polymer has specific solubility at equivalent pH is beneficial for colon targeting however, a pH dependant polymer can shield a formulation in the stomach, and proximal small intestine, it start to dissolve in the colon (Ashish, 2018). A multiple range of mechanisms have been developed to attain colon targeting of drugs. Mostly used mechanisms are to coat the formulation using natural or synthetic polymer (M. Prathap et al., 2014). In this mechanism, the drug is intended in the core of the formulation which is coated with PH sensitive Polymer. The first coating is an acid soluble polymer and outer coating is an enteric polymer (Chaurasia and Jain, 2008). the core of formulation is comprised of the API and excipients. When the drug pass through the GI tract the formulation does not degraded or release in the stomach due to enteric coating, but the enteric coating will dissolve in the small intestine, where the PH is above 6. When the drug reaches to the colon, the polysaccharide into organic acid. This lower the PH of the surrounding system and cause the dissolution of the surrounding system and results in dissolution of the surrounding system acid soluble coating and subsequent drug release (Neha and S.L., 2013). The most commonly used pH dependent polymer are derivatives of acrylic acid and cellulose (Nishant and Dr. R.C., 2012).
|
S.No. |
Polymer |
Threshold PH |
|
1 |
Eudragit S-100 |
7 |
|
2 |
Eudragit PL-100 |
6 |
|
3 |
Eudragit Fs30D |
>7 |
|
4 |
Hydroxypropylmethy Cellulose phthalate |
>5.5 |
|
5 |
Shellac |
7 |
|
6 |
Eudragit L100-55 |
5.5 |
Table No. 05- Showing different PH sensitive polymers and their threshold pH release (Gurmeet et al., 2012).
B) Delayed (Time controlled release system) release drug delivery system.
Time dependent / Controlled release system such as sustained or delayed release dosage form are also very promising drug release system However due to potentially large variations gastric emptying time of dosage forms in humans, in these approaches, Colon arrival time of dosage forms cannot be accurately predicted, resulting in poor colonial availability (Threveen et al., 2011). The strategy in designing time released systems is to resist the acidic environment of the stomach and to undergo a lag time of predetermined span of time, after which release of drug take place the lag time in this case is the time required to transit from the mouth to colon a lag time of 5 hours is usually considered sufficient since small intestine is about 3-4 hours, which is relatively constant and hardly affected by the nature of formulation administered. (Mukesh et al., 2013).
Disadvantages of this system are-
• Gastric emptying time varies markedly between subjects or in a manner dependent on type and amount of food intake.
• GIT movement would result in the change in gastrointestinal transit of the drug.
• Accelerated transit through different region of the patient with the carcinoid syndrome, diarrhea and ulcerative Colitis. (M. Prathap et al., 2014)
C) Microbially triggered system
Targeting of drugs to the colon is effective and safe for the treatment of various colonic disease. These diseases are often poorly and inefficiently managed either by oral route in which oral drugs are largely absorbed before they rich the colon or by the rectal route of administration which is less acceptable. Microbial triggered delivery system is most convenient and highly site specific approach for targeting drug to the colon than all targeting system. Drug release when the polysaccharides are degraded by the bacteria of the colonic micro flora (Rupali et al., 2015). The upper part of GIT i.e the stomach and the duodenum has a micro flora of less than 103 – 104 CFU /ml. the micro flora of Colon on the other side is is the range of 1011-1012 CFU/ml consisting mainly of anaerobic bacteria. E.g. Bactericides, Bifid bacteria, Eubacteria, Clostridia Enterococci, Enterobacteria ect. Vast number of enzymes like glucuronidase, xylosidase, arabimosidas, arabimosidase, galactosidase, nitroteductase, azoreductase, deaminase and urea dehydroxylase are produce by micro flora Because of the presence of these biodegradable enzymes, only in the colon, the use of bacterial degradable polymers for colon specific drug delivery seems to be a more site specific approach as compared to other approaches (M. Mallikarjuna et al., 2012).
D) Pro-drug approach for drug delivery to colon
Pro-drug are pharmacologically inactive chemical derivatives that could be used to alter the physiochemical properties of drug in a temporary manner or to increase their usefulness and to decrease associated toxicity. A pro-drug is a compound formed by chemical modification of biologically active compound that will liberate the active compound in vivo by enzymatic or chemical processes (Vimal and Vikram, 2012). Pro-drug is the main approach of microbial triggered drug delivery system is system in which the drug release from the formulation is triggered by the micro flora present in the gut. (M. Prathap et al., 2014). Biotransformation is carried out by a variety of enzymes, mainly of bacterial origin, present in the colon. The enzymes that are mainly targeted for colon drug delivery include azoreductase-galactosidase, B-xylsidase, nitroreductases. Generally, a pro-drug is successful as a colon drug carrier of it is hydrophilic and bulky, to minimize absorption from the upper GI tract and once in the colon, it is converted into a more lipophilic drug molecule that is then available for absorption (Threveen et al., 2011). A clinical study ha shown clear evidence that B-cyclodextrins are poorly digested in the small intestine but are almost completely degraded by the colonic microflora (C. Tuleu et al., 2001).
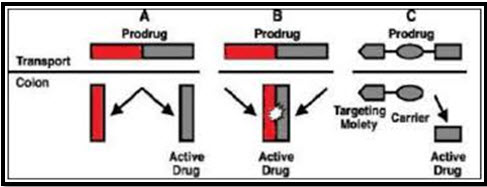
Diagram 02- Pro-drug approach
|
Drug investigated |
Carrier |
Linkage hydrolgsed |
|---|---|---|
|
5-ASA |
Azo conjugates, Sulphapyridine (SP) |
Azo-linkage |
|
Naproxane |
Dextran conjugate |
Ester linkage |
|
Dexamethasone |
Dextran conjugate |
Using spacer |
|
5-ASA |
Azo – Conjugates, Glycine |
Amide linkage |
Table No. 06- Produrg evaluated for colon specific drug delivery (Threven et al., 2011)
E) Polysaccharide Based delivery system-
Polysaccharide based delivery system is the other form microbial triggered drug delivery system (M. Prathap et al., 2014). the inability of GIT enzymes to digest certain plant polysaccharides is taken as an advantage to develop colon specific drug delivery system. The drug is embedded in the matrix core of the biodegradable polymer by compressing the blend of active drugs, a degradable the blend of active drugs a degradable polymer and additives (Ravi et al., 2009). Many natural polysaccharides such as chondroitin sulphate, pectin, dextran and more particularly guar gum etc. has started with basis of investigation for their potential in innovating colon specific drug delivery. These are led to break down by the colonic micro flora to simple saccharides (Vikash et al., 2015). They can be easily modified chemically, biochemically and are highly stable, safe, non-toxic, and hydrophilic and in addition are biodegradable. The use of naturally occurring polysaccharides is attracting a lot of attention for drug targeting the colon since these polymers are found in abundance, have wide availability, inexpensive and are available in a variety of structures with varied properties (Amresh et al., 2016).
2- Newly developed approaches for CDDS-
A) Pressure Controlled drug-delivery system
As a result of peristalsis, higher pressures are encountered in the colon than in the small intestine Takaya et al. developed pressure controlled colon- delivery capsules prepared using ethyl cellulose, which is insoluble in water (Anil and Betty, 2010). Peristaltic motion causes the luminal pressure of the large intestine to increase more than that of the small intestine because its contents are more viscous due to the reabsorption of water. Several studies have been carried out to utilize the colonic luminal pressure to pressure to develop colon- specific drug delivery systems (S. Amidon et al., 2015). the pressure controlled drug delivery system consists of a capsule in which the drug is present. These gelatin capsules are coated with water insoluble polymer like ethylcelluose on their inner side the drug is introduced into the capsule along with suppository base dissolves at body temperature the water from intestinal contents in absorbed resulting in increased viscosity which leads to an increase in the pressure in the capsule expels the drug into the colon (T. Nalanda and Prashant, 2015).
B) Pulsatile colon targeted drug delivery
i) Pulsin Cap system
ii) Port system
i) Pulsincap System-
Pulsincap was developed by R.R. Scherer. International Corporation (Michigan) (Vikash et al., 2011). In this system the formulation is developed in a capsule form. The plug placed in the capsule controls the release of the drug. Swellable hydrogels are used to seal the drug contents. The capsule, gets swelled when it comes in contact with the dissolution fluid and after a lag time the plug gets pushed off from the capsule and the drug will be released (Amit et al., 2015). Polymers such as different grades of hydroxyl propyl methyl cellulose (HPMC), poly methyl methacrylate and polyvinyl acetate are used as hydrogel plugs. The log time is controlled by the lenth and point of intersection of the plug in the capsule body (Rama et al., 2014).
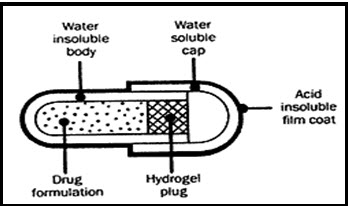
Diag. 03- PulsinCap system
ii) Port System:-
The port system was developed therapeutic system research laboratory Ann Arbor, Michigan, USA, and consist of a capsule coated with a semi-permeable membrane, inside the capsule was an insoluble plug consisting of osmotically active agent and the drug formulation (Vipul and Moinuddin, 2012). When this capsule came in contact with the dissolution fluid, the semi-permeable membrane allowed the entry of water, which caused the pressure to develop and the insoluble plug expelled after a lag time. This system avoids second time dosing, which was beneficial for school children during daytime (J. Ali et al., 2006). The lag time is controlled by coating thickness. The system showed good correction in lag time of in-vitro and in-vivo experiments in humans (V.N.L. Sirisha et al., 2012).
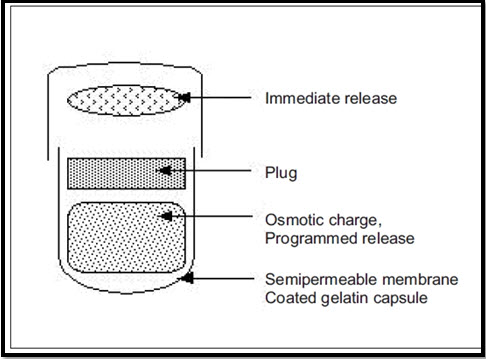
Diag. 04- Plan of Port System
(C) CODES technology (combination of PH dependent and microbially triggered CDDS)
CODES was designed to overcome the problems associated systems. In this system both the pH and microbially triggered approach are utilize the controls the drug release at desired location (Vipin et al., 2012). It has been developed by utilizing a unique mechanism involving lactulose, which acts as a trigger for site specific drug release in the colon (Vinay et al., 2012). It consists of core tablets coated with three layer of polymer coatings. The first coating is an acid soluble polymer (eudragit) and outer layer is enteric with a HPMC barrier layer in between to prevent any possible interaction between the oppositively charged polymers (Bhushan et al., 2012). The premise of the technology is that the enteric coating protects the tablet while it is located on the stomach and then dissolves quickly following gastric emptying. The acid soluble material coating then protects the preparation as it passes through the alkaline PH of the small intestine. Once the tablet arrives in the colon, the bacteria enzymatically degrade the polysaccharide (lactulose) into organic acid. This lowers the PH surrounding the system sufficient to affect the dissolution of the acid soluble coating and subsequent drug release (Anil and Betty, 2010). The other polysaccharide that are used along with the drug in the core. Tablet are mannitol, maltose etc. The bacteria present in the colon are responsible for the degradation of polysaccharides that are released from the core tablet (Brahma P. et al., 2010).

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
(D) Osmotic Controlled Drug Delivery (OROS-CT)
Osmotic controlled drug delivery (Alza corporation) is used as a once or twice a day formulation for targeted delivery of drugs to the colon. The OROS-CT can be a single osmotic agent or it can be comprised of as many as five to six push pull osmotic unit filled in a hard gelatin capsule(Brahma et al., 2010). After coming in contact with GI fluids, the gelatin capsule dissolves and the enteric coating prevents the entry of fluids from stomach into the system (Roop et al., 2014). The osmotic pumping action results when the coating dissolves in the high pH environment (pH>7) of the small intestine and the drug is delivered out of the orifice at a rate controlled by the rate of water transport across the membrane (K. Raja et al., 2015).
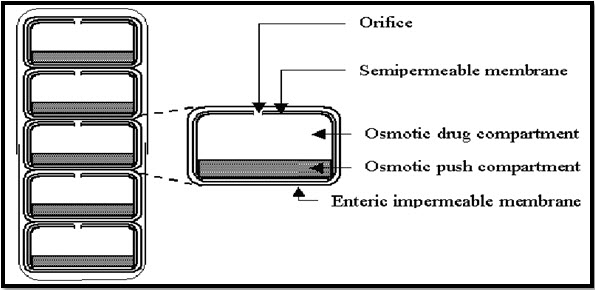
Diag. 06- OROS-CT
(E) Multiparticulate system based drug delivery
Multiparticulate drug delivery systems are mainly oral dosage forms consisting of a multiplicity of small discrete units, each exhibiting some desired characteristics (NS Dey et al., 2008). The various advantages of this systems are increased bioavailability, reduce risk of local irritation, reduce risk of systemic toxicity. The various multiparticulate approaches include pellets, micro particles, granules and nano-particles (Nalanda and Prashant, 2015). To administer or to recommend total dose these subunits are compressed into a tablets or filled into a sachets or encapsulated. These are capable of protecting the drugs from the chemical and enzymatic degradation in GIT resulting in an increase in their stability and absorption of through the intestinal epithelium (Bipin and Jagdish, 2013).
(F) Azo hydrogel
The pH sensitive monomers and azo cross linking agents in the hydrogel produce the colon specificity. During their passage through GIT these hydrogels swell as the pH increase. This swelling of hydrogels cleaves the cross links in the hydrogel network causing the release of drug entrapped in the hydrogel (Rama Krishna et al., 2014). These hydrogels are prepared by cross linking polymerization of N-substituted (Meth) acrylamides, N- tert- butyl acrylamide and acrylic acid with 4, 4-di (methacryloylamino) azobenzene as cross linking agents. The degradation rate of hydrogen is associated with the degree of swelling and inversely proportional to the cross linking density (Nalanda and Prashant, 2015).
(G) Pro-biotic approach:-
The pro-biotic approach is one of the modern technique for colon targeting. In this approach three components are desirable namely probiotic strain, microbial digestible carrier and triggered temperature. Pro-biotic stains include inactive micro flora like bifidobacterium and lactobacillus species. At body temperature, these strains triggered to be active and start digesting the carrier and ultimately release the drug at desired place. This approach gains success in colon drug delivery system because these conditions are only available in colon (Prashant et al., 2010).
EVALUATIONS
In Vitro Evaluation-
No standardized evaluation technique is available for evaluation of CDDS because an ideal in vitro model should posses the in vivo conditions of GIT such as PH, volume, stirring, bacteria enzymes, enzyme activity and other components of food. Generally these conditions are influenced by the diet and physical stress and these factors make it difficult to design a standard in vitro model. The in vitro evaluation of colon targeted drug delivery systems includes the in- vitro dissolution study and in vitro enzymatic test (Rama Krishna et al., 2014).
i) In-Vitro dissolution Test- The dissolution testing is done using the conventional basket method. The dissolution testing is done in different buffers to characterize the behavior of formulation at different PH level (M. Prathap et al., 2014). The different media that are used for the dissolution testing of colon targeted drug delivery are PH 1.2 to stimulate gastric fluid, PH 6.8 to simulate small in testing, PH 7.4 to simulate large intestine. The colon targeted drug delivery are tested for 2 hour in 0.1N HCl, 3 hour in PH 6.8 phosphate buffer and finally at PH 7.4 phosphate buffer. Buffers of the above PH are prepared to evaluate the colon targeted drug delivery system. (Danda and Chandan, 2013)
ii) In vitro enzymatic test- These are 2 tests for the in- vitro enzymatic test.
• The carrier drug system ia incubated in fermenter containing suitable medium for bacteria The amount of drug release at different time intervals is determined.
• Drug released study is performed in buffer medium containing enzymes pectinase, dextranase or rat or guinea pig or rabbit cecal contents. The amount of drug released in a particular time is directly proportional time is directly proportional to rate of degradation of polymer Carrier (Archana and Divya, 2016).
In – vivo evaluation-
The in-vivo evaluation of the CDDS is done in dogs guinea pigs, rats and pigs as they resemble the anatomic and physiological conditions micro, flora of human GIT the distribution of various enzymes in GIT of rat and rabbit is comparable to that in human. (K. Malleshwari and Vijaya, 2016)
CONCLUSION
Colon targeted drug delivery system provide benefits of local and systemic effects. The main advantage of colon drug delivery system is that the colon offers a long transit time, near neutral PH, reduced enzymatic activity and increased responsiveness to absorption to absorption enhancers. The most critical challenge in CDDS is to preserve the formulation during its passage through the stomach and about six meters of small intestine. After seeking the limitation of different approaches, researchers invented various novel approaches. The novel approaches like pressure controlled drug delivery system, pulsincap system, port system, CODES, multiparticulatesystem, probiotic approach and azo hydrogels are more specific compared to primary approaches. Both natural and biodegradable polymers are used for the colon specific delivery of the drug. The need of today's business and patient community is to identify the appropriate approach that con result in the delivery of drugs in safe effective and less expensive manner with minimum fluctuation in term of release of drugs at target site.
REFERENCES
1. Agarwal Vipin K., Gupta Amresh, Chaturvedi Shashank, Khan Farheen (2016); "Polysaccharide: carrier in colon targeted drug delivery system"; MIT International Journal of Pharmaceutical Sciences; 2(2); Page no. 1-9.
2. Ali J., Arora Shweta, Ahuja Alka, Baboota Sanjula, Qureshi J. (2006); "Pulsatile drug delivery system : An approach for controlled drug delivery; International Journal of Pharmaceutical Sciences; 68(3) ; Page no. 295-300.
3. Arora Vimal, Lohar vikram (2012); "Prodrug approach to better drug delivery”; International Journal of Pharmacentical Research; 4(1); Page no. 15-21.
4. Campieri Massimo, Corbelli Claudio, Gionchetti Paolo, Brignola Corrado (1992); “Spread and distribution of 5-ASA colnic foam and 5-ASA enema in patient with ulcerative colitis”; Digestive diseases and secienes; 37(12); Page no. 1890-97.
5. Challa Threveen, Vynala Vinay, Allam Krishna Vamshi (2011); “Colon specific drug delivery system: A review on primary and novel approaches"; International Journal of Pharmaceutical Sciences Review & Research; 7(2); Page no. 171 - 181.
6. Chaudhuri Sujit K. (2014); “Concise Medical Physiology"; New Central Book Agency (p) Ltd., India; reprint edition; Page no 126-127.
7. Chourasia M.K. Jain S.K (2004); "Polysaccharides for colon targeted drug delivery”; Drug Delivery and Therapeutics; 11(2); Page no. 129-148.
8. Dey NS, Majumdar S., Rao MED (2008); " Multiparticulate drug delivery System for controlled release”; Topical Journal of Pharmaceutical Research; 7(3); Page no. 1067-1075.
9. Dhir kushal, kahlon Harminder pal singh, kaur Sukhbir (2013); " Recent approaches for colon targeted drug delivery system"; International Journal of Pharmaceutical Chemical and Biological Sciences; 3(2); Page no. 360-371.
10. Dhyani Archana, Jugal Divya (2016); "Colon targeted drug delivery system: A review on primary and novel approaches”; International Journal of Pharmaceutical Science and Research; 1(5); Page no. 90-97.
11. Ghandhi Bipin, Baheti Jagdish (2013); "Multiparticulates drug delivery systems: A Review”; International Joural of Pharmaceutical and Chemical Sciences; 6(10); Page no. 1620-1626.
12. Ghosh Prashant Kumar, Gupta Vipin B., Gondoliya Bhavik, Rathore Mahendra Singh (2010); "Probiotic-assisted colon-specific delivery of diclofenac sodium from guarfum matrix tablets : In vitro evaluation"; Asian Journal of Pharmaceutics; Page no. 173-178.
13. Gouda M. Mallikarjuna, Shafaraya A. Ramakrishna, kumar SM Shanta (2012); "Formulation and evaluation of colon specific microbial degradable matrir tablet using sterculia gum as carrier”; International Current Pharmaceutical Journal; 1(11); Page no. 376-383.
14. Gupta Akhil, Mittal Anuj, Gupta Alok Kumar (2011); "Colon targeted drug delivery system A review"; Russian Jornal of Biopharmaceutics; 3(4); Page no. 3-13.
15. Gupta Brahma P., Thakur Navenct, Jain Nishi P., Banweer Jitendra, Jain Surendra (2010); "Osmotically controlled drug delivery system with associated drugs”; J. Pharmaceutical Sci; 13(4); Page no. 571-588.
16. Gupta Roop N., Gutpa Rakesh, Dasniwal Pawan k., Rathore Garvendra S. (2014), "Osmotically controlled oral drug delivery- A Reviw”; International Journal of Pharmaceutical Sciences; 1(2); Page no. 269-275.
17. Gupta Vinay K., Gnanaraj G., Kothiya Preeti (2012); "A review article on colonic targeted drug delivery system"; The Pharma Journal; 1(7); Page no. 14-24.
18. Halsa M, Penttinen T, verki P, Jurjenson H, Marvol M (2001); "Time controlled released pseudoephedrine tablets bioavailability and in vitro/ in vivo correlations"; Pharmazie; 56(9); Page no. 718-723.
19. Jain Ashish (2018); "Colon targeting using pH Sensitive material"; Advanced Research in Gastroenterology & Hepatology; 8(5); Page no. 1-3.
20. Jain N.K (2001); "Advanes in controlled & novel drug delivery”; CBS publishers and distributors; 1st edition; Page no 86-90.
21. Jain Vikash, Jain Deepika, Raturi Richa, Bansal Praveen, Singh Ranjit (2011); "Recent technologies in pulsatile drug delivery systems”; Bio matter; 1(1); Page no. 57-65.
22. Jain Vikash, Shukla Neha, Mahajan SC. (2015); "Polysaccharides in colon specific drug delivery"; open Access text.
23. Jandhyala Sai Mansa, Talukdar Rupjyoti Subramanyam Chivkula, Vayywa Harish, sasikal Mitnala, Reddy D. Nageshwar (2015); “Role of the normal gut microbiota”; World Journal of Gastroenterology; 21(29); Page no- 8787- 8803.
24. Kinget Renaat, Kalala W., Vervoort L, Van Den Mooter G (1998); “Colonic drug targeting”; Journal of Drug Targeting; 6(2); page no. 129 -149.
25. Kolte Bhushan prabhakar, Tele Kalyani V., Mundhe Vinayak S., Lohoti Sandeep S. (2012); "Colon targeted drug delivery system A novel perspective"; Asian Journal of Biomedical and Pharmaceutical Science; 2(14); Page no. 21-28.
26. Kumar Amit, Aggarwal Geeta, Harikumar S.L. (2015); "Colon specific drug delivery by Ph Sensitive polymers and pulsatile drug delivery system”; Indo Global Journal of Pharmaceutical Sciences; 5(1); Page no. 6-11.
27. Kumar Ravi, Patil M.D, Patil Sachin R., Paschapur Mohesh S. (2009); "Polysaccharieds based colon specific drug deliery : A Review"; International Journal of Pharmtech Research; 1(2); Page no. 224-346.
28. Kumar Vipin, Verma Surendra, Mishra D.N., Singh S.K. (2012); "Colon targeted drug delivery: current and novel perspectives”; International Journal of Pharmaceutical Sciences and Research; 3(5); Page no. 1274-1284.
29. Malleswari K., Ratna Vijaya (2016); "Colon targeted drug delivery review and novel approaches”; World Journal of Pharmacy and Pharmaceutical Science; 5(5); Page no. 1636-1650.
30. Nemade Moheah S, Chaudhari Rajesh Y, Potil Vijay R (2014); "Novel approaches to colon targeted drug delivery system A Review”; Research and Reviews in Pharmacy and Pharmaceutical Science ; 3(2); Page no. 63-69.
31. Nugent SG, kumar D., Ramptan DS, Evans DF (2001); "Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with amino salicylates and other drugs"; BMJ Journal and GUT; 48(4); Page no. 571-577.
32. Padmala Rama krishan Reddy, kondeti Ranjith Reddy, Rajushalem, Mulpuri Kranti Sri (2014); "Colon drug delivery system : A novel perspective"; World Journal of Pharmacy and Pharmaceutical Sciences; 3(9); Page no. 212-227.
33. Patel Vipul P, Soniwala Moinuddin M (2012); "Pulsatile drug delivery system for treatment of various inflammatory disorder : A Review”; International Jouranl of Drug Development and Research; 4(3); Page no. 67-87.
34. Philip Anil K., Philip Betty (2010); "Colon targeted drug delivery systems a review on primary and novel approaches"; Oman Medical Journal; 25(2); page no 79-87.
35. Prathap M, Gulshan M.D., Rao N. Rama (2014); "Colon: targeted drug delivery system A Review"; International Jouranl of Research in Pharmaceutical and Nano Sience; 3(5); Page no. 429-437.
36. Rajpurohit H, Sharma P, Sharma, Bhandari A (2010); “Polymers for colon targeted drug delivery system”; Indian Journal of Pharmaceutical Sciences; 72(6); Page no. 689-969.
37. Rangari Nalanda T, Puranik Prashant K (2015); "Review on recent and novel approaches to colon targeted drug delivery systems"; International Journal of Pharmacy and Pharmaceutical Research; 3(1); Page no. 167 - 186.
38. Rao K. Raja, Naik V. Vasu, Rao A. Anka, Rajesh A (2015); " Osmotic controlled drug delivery system: A review"; International Journal of Pharma and Chemical Research; 1(1); page no, 31- 37.
39. Rathod Shruti (2007); “Colon targeted pulsatile drug delivery a review”; Pharma Info Net; Page no. 1-11.
40. Ratnaparkhi Mukesh P., Somvanshi Fatte Singh U., Pawar Sham A., Chaudhari Shilpa P, Gupta Jyoti P., Budhavakant Kalyani A (2013); “Colon targeted drug delivery system"; International journal of Pharma Research and Review; 2(8); page no. 33-42.
41. Sears Cynthia L., Garrett wendy S. (2015); "Cell Host & Microbe"; PMC; 15(3); Page no. 317-328.
42. Seth Amidon, Brown Jack E., Dave Vivek S. (2015); "Colon targeted oral drug delivery system design trends and approach"; AAPS Pharma SciTech; 16(4); Page no 731-741.
43. Sharma Neha, Harikumar S.L (2013); "Polymer for colon targeted drug delivery system"; International Journal of Drug Development and Research; 5(1); Page no. 21-31
44. Singh Amritpal, Sharma Ankush, Pooja, Anju (2014); "Novel approaches for colon targeted drug delivery system"; International Journal of Research and Development in Pharmacy and Life Science ; 3(2); Page no,. 877-886.
45. Singh Gurmeet, Kumar Dinesh, Singh Mankaran, Sharma Deepak, Kaur Sukhbir (2012); "Emerging techniques and challenges in colon drug delivery system; journal of Applied Pharmaceutical Science; 2(3); Page no. 139-147.
46. Singh Nishant, Khanna DR. R.C. (2012); "Colon targeted drug delivery system-A potential approach"; The Pharma Innovation; 1(1); page no 40-47.
47. Sirisha V.N.L., Namrata M., Sruthi B., Harika I; Kumar P. Kiran, Rao Y. Kiran Kumar, Pranavi K, (2012); "Pulasatile drug delivery system –A Review; International Jouranl of Pharmaceutical Research and Allied Sciences; 1(3); Page no, 13-23.
48. Sreelatha Danda, Brahma Chandan K (2013); “Colon targeted drug delivery- A review on primary and novel approaches"; Journal of Global Trends in Pharmaceutical Sciences; 4(3); Page no. 1179-83.
49. Tiwari Gaurav, Tiwari Ruchi, Wal Pranay, Wal Ankita, Rai K. Awani (2010); “Primary and novel approaches for colon targeted drug delivery- A Review; International Journal of Drug Delivery; 2; Page no. 01-11.
50. Tuleu C., Andrieux C., Cherbuy C, Darcyvrillon , B., Duee, P.H., Chaumeil, J.C. (2001); "Colonic delivery of sodium butyratevia oral route : acrylic coating design of pellets and in-vivo evaluation in rats"; Methods Find Exp Clin Pharmacol; 23(5); Page no, 245-253.
51. V.V Prasanth, R. Jayabrakash, Mathew Sam (2012); "Colon specific drug delivery system a review on various pharmaceutical approaches"; Journal of Applied Pharmaceutical Sciences; 2(1); Page no. 163-69.
52. Vagare Rupali, Doijad Rajendra, Shete Amol, Jagtab Rajesh, Mohit Poonam, Sajane Sachin (2015); "Microbial triggered colon targeted compression coated tablets of tenixicam : formulation and evaluation"; Journal of Drug Delivery and Therapeutics; 5(1); Page no. 75-81.
53. Waugh Anne, Grant Allison (2010); "Ross & Wilson Anatomy physiology and pathophysiology in health and illness; Churchill Livingstone; 11 edition; Page No. 297-298.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









