{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Pushpa latha E*1, Sailaja B2
1,2 Dept. of Pharm. Analysis & Quality Assurance, Institute of Pharmaceutical Technology, Sri Padmavathi Mahila Visvavidyalayam, Tirupati, Andra Pradesh, India-517502
ABSTRACT
Rizatriptan is a selective serotonin receptor agonist of the 1B and 1D sub types used in the acute treatment of migraine attacks. Effective analytical procedures are required for qualitative and quantitative determination of a drug. An extensive literature survey has been conducted in various analytical and pharmaceutical journals and the analytical methods which were developed and used for estimation of Rizatriptan in single or in combination with other drugs in bulk drugs, pharmaceutical formulations, biological fluids, stability indicating and impurity profiling methods have been reviewed. Various analytical methods used for the estimation of Rizatriptan reviewed in this paper includes Ultraviolet spectrometry, Visible spectrometry, High performance thin layer chromatography, High performance liquid chromatography, Liquid chromatography- mass spectrometry.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2626
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 11 Received On: 23/10/2018; Accepted On: 24/10/2018; Published On: 01/11/2018 How to cite this article: latha, P. and Sailaja, B. 2018. A Review on Analytical Methods for the estimation of Rizatriptan, an Antimigraine Drug. PharmaTutor. 6, 11 (Nov. 2018), 36-52. DOI:https://doi.org/10.29161/PT.v6.i11.2018.36 |
INTRODUCTION
Migraine is a common recurring headache of moderate to severe intensity that interferes with normal functioning i.e., gastrointestinal, neurological and autonomic symptoms [Barbara G Wells et al., 2005]. Migraine attack consists of an initial visual disturbance (aura), in which a flickering pattern, followed by a blind spot. This visual disturbance is followed for about 30min, later by a severe throbbing headache, often accompanied by photophobia, nausea, vomiting and prostration which lasts for several hours [Rang H P et al., 2012]. Migraine is a common condition affecting 5% of men and 15% of women [Clive Page et al., 2009]. Migraine headaches are subclassified according to the presence or absence of aura symptoms. Most persons who suffer from migraine do not experience aura symptoms [Brain K. Alldredge et al., 2013].
Pathophysiology:
Migraine may be due to
i. Neuronal dysfunction characterized by a wave of depressed electrical activity that advances across the cerebral cortex i.e., cortical excitation.
ii. Trigeminal afferents from the dural vasculature are activated and sensitized by the local release of neuropeptides and inflammatory mediators.
iii. Imbalance in the activity of serotonin containing neurons in brainstem nuclei that modulate cerebral vascular tone and nociception. This imbalance may result in vasodilation of intracranial blood vessels and action of trigemino vascular system [Barbara G Wells et al., 2005, David E. Golan et al., 2012].
• 5-hydroxytryptamine or 5-HT or serotonin is a neurotransmitter which is the target for many of the drugs used to treat psychiatric disorders such as depression and it play critical roles in modulating mood, sleep-wake cycle, motivation, pain, perception, neuroendocrine function [David E. Golan et al., 2012].
• Serotonin is synthesized from the aminoacid tryptophan by the enzyme tryptophan hydroxylase which converts tryptophan to 5-hydroxytryptophan. Aromatic L-aminoacid decarboxylase then converts 5-hydroxytryptophan to serotonin. These enzymes are present throughout the cytoplasm of serotonergic neurons, both in cell body and in cell processes [David E. Golan et al., 2012].
• Abnormlities in 5-HT/serotonin activity play a role in migraine headache. Plasma 5-HT levels decrease by nearly half during a migraine attack, with a corresponding risk in the urinary excretion of 5-hydroxyindole acetic acid, the primary metabolite of 5-HT [Brain K. Alldredge et al., 2013].
Drugs used for treatment of Migraine [Tripathi K D. 2006]:
1. Mild: Simple analgesics/ NSAIDs such as Aspirin, Naproxen sodium, Tolfenamic acid, Indomethacin, Mefenamic acid etc. with antiemetic.
2. Moderate: Combination of NSAIDs such as Aspirin with codeine, Aspirin with oxycodone. Combiination of Aspirin, Acetaminophen and caffeine was found to be more efficient. Combination of Acetaminophen, Caffiene and Butabital is given only for short periods since the combination can cause dependence [Clive Page et al., 2009].
3. Severe: Ergot alkaloids, Triptans.
Ergotamine is an adrenergic agonist with affinity for 5-HT1 receptors, stimulation of which leads to vasoconstriction. These drugs are contraindicated in patients with coronary artery or peripheral vascular disease [Clive Page et al., 2009].
TRIPTANS:
Triptans are 5-HT1 receptor agonists, very active against mild to moderate attacks of migraine. This is the first choice of specific drugs in the symptomatic treatment of migraine.
First Generation Triptans: Sumatriptan is the first generation triptan that is approved for use.
Second Generation Triptans: Zolmitriptan, Naratriptan, Rizatriptan, Almotriptan, Frovatriptan, Elatriptan [Waldman and Terzic 2009].
Rizatriptan
Drug Profile [wikipedia.com]
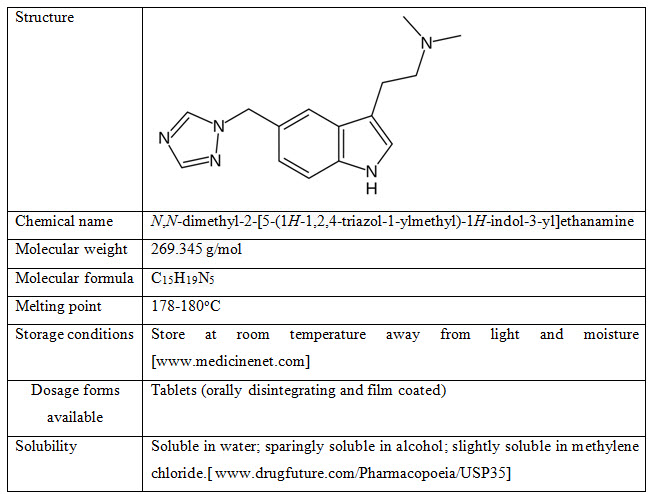
Mechanism of Action:
It works by narrowing blood vessels in the brain, stopping pain signals from being sent to the brain, and blocking the release of certain natural substances that cause pain, nausea, and other symptoms of migraine [nlm.nih.gov/medlineplus].
Pharmacokinetics:
Rizatriptan is readily absorbed from the gastrointestinal tract. The biological half-life of Rizatriptan is approximately 2-3hours. After absorption, rizatriptan (oral) is rapidly eliminated in urine[drugs.com].
Analytical Methods for Rizatriptan
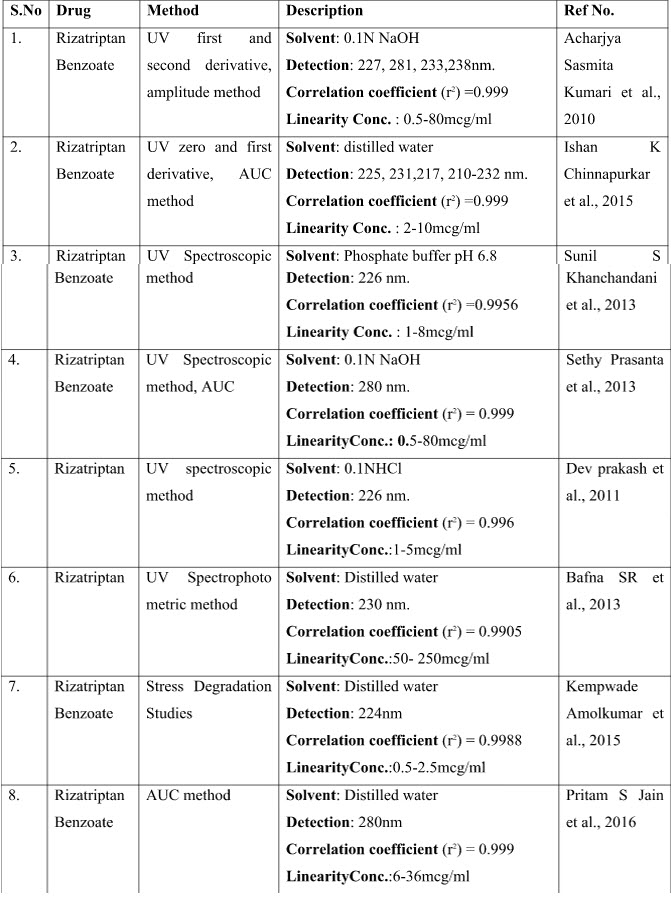
Various analytical methods have been developed for the estimation of Rizatriptan in single and in combination which includes Spectrophotometric, HPLC, HPTLC, LC-MS methods.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
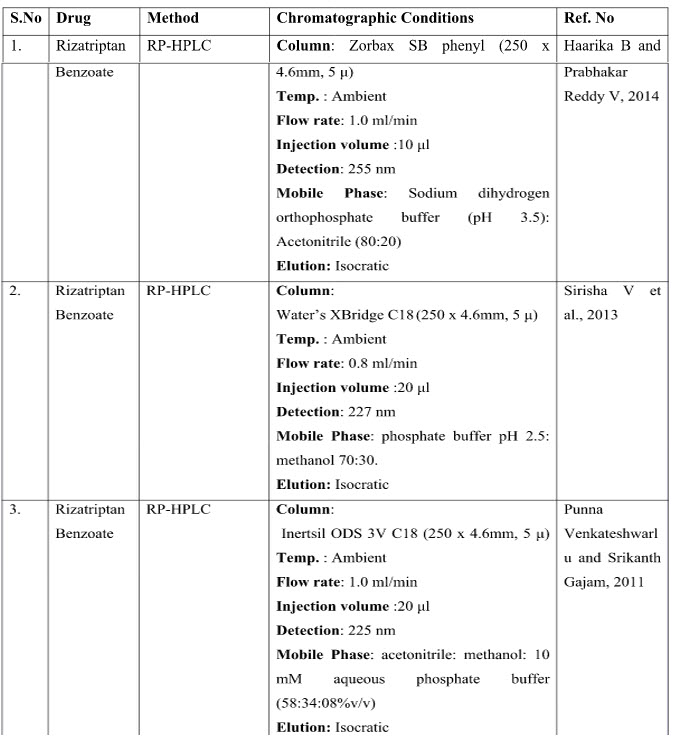
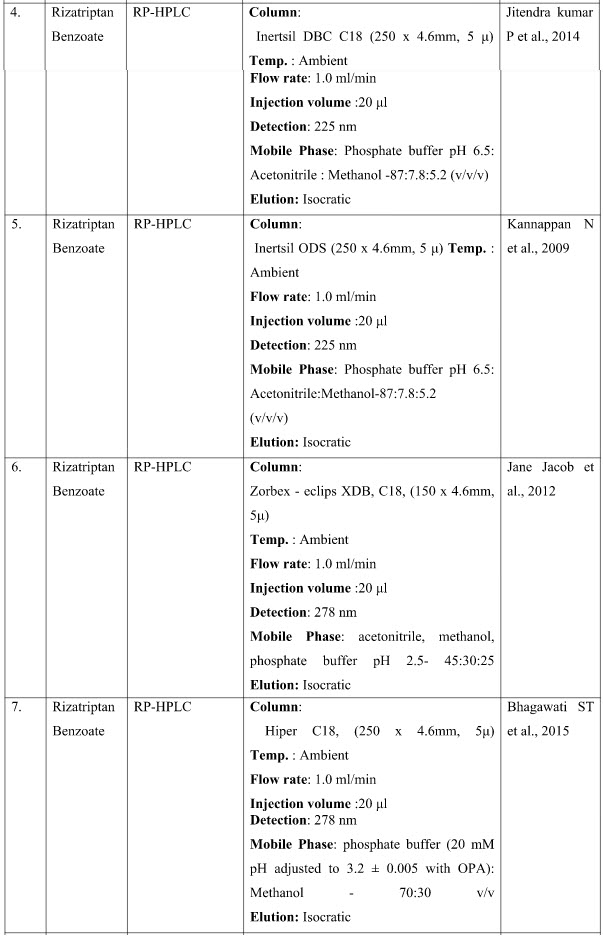
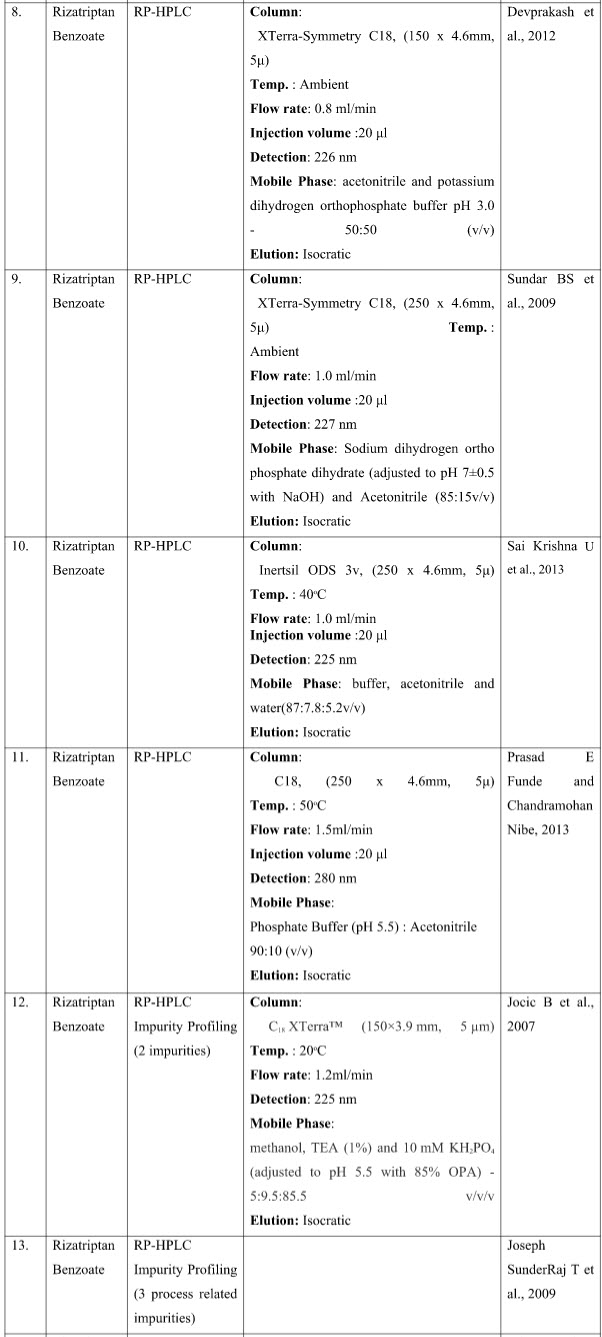
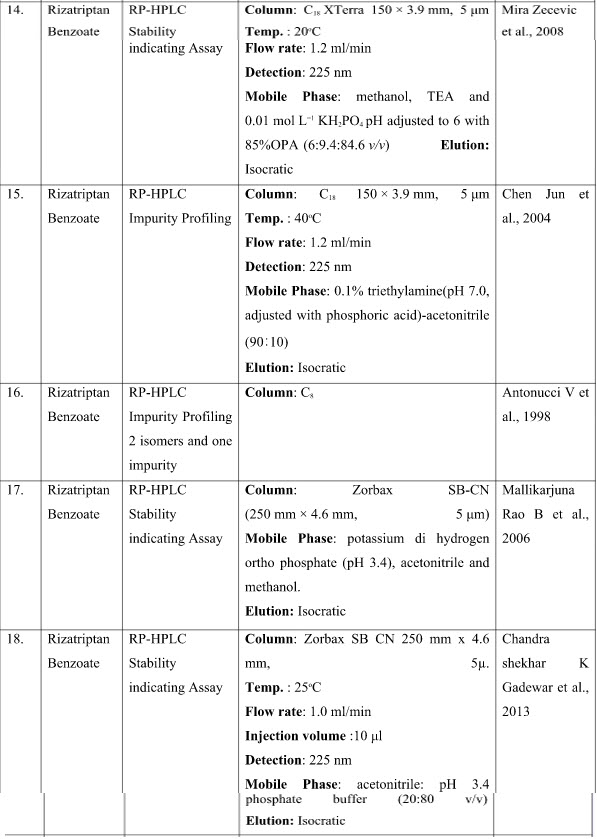
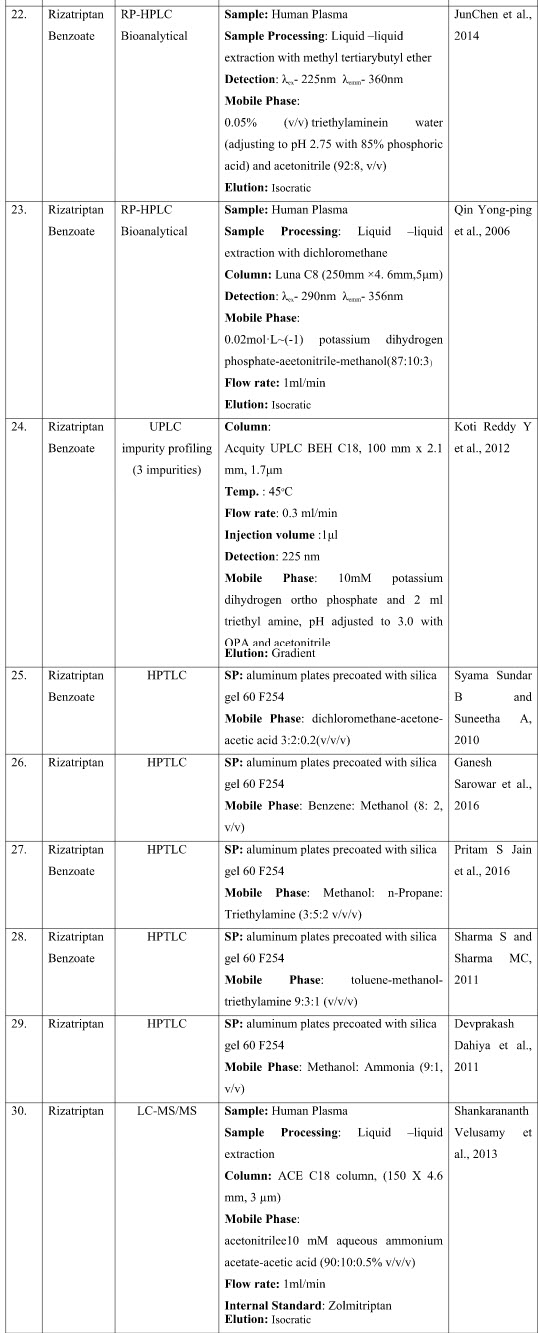
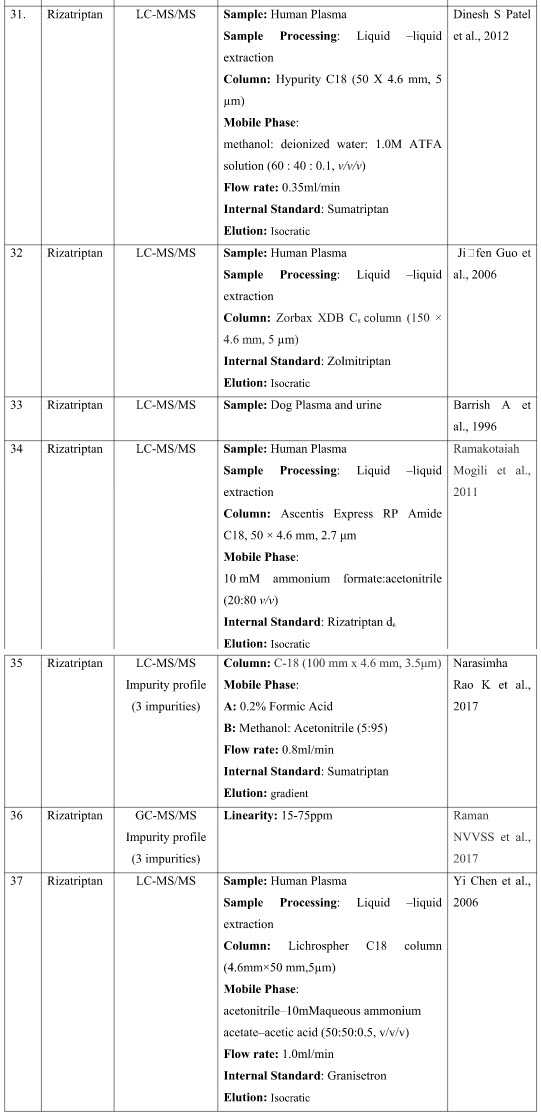
CHROMATOGRAPHIC METHODS

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
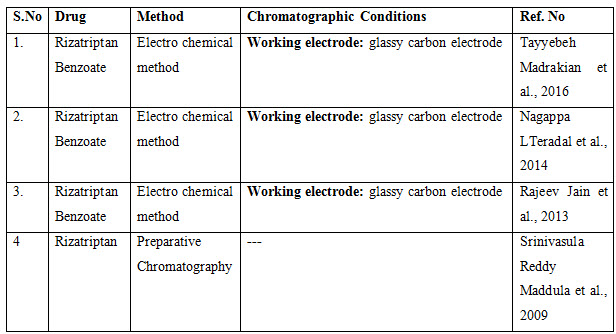
OTHER METHODS
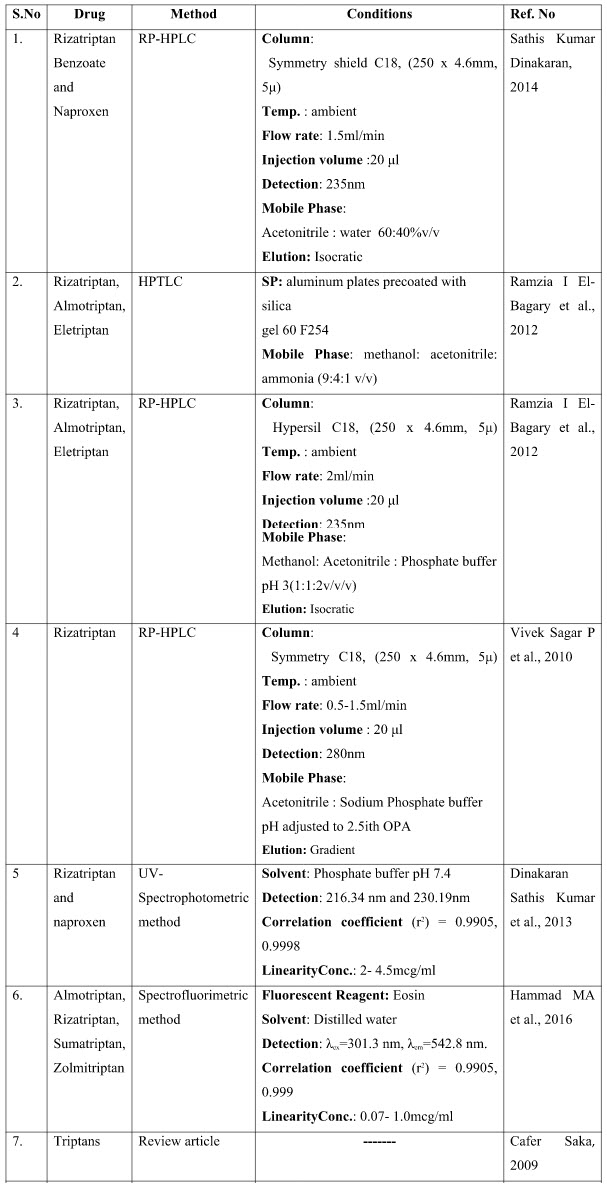
ANALYTICAL METHODS FOR RIZATRIPTAN IN COMBINATION WITH OTHER DRUGS
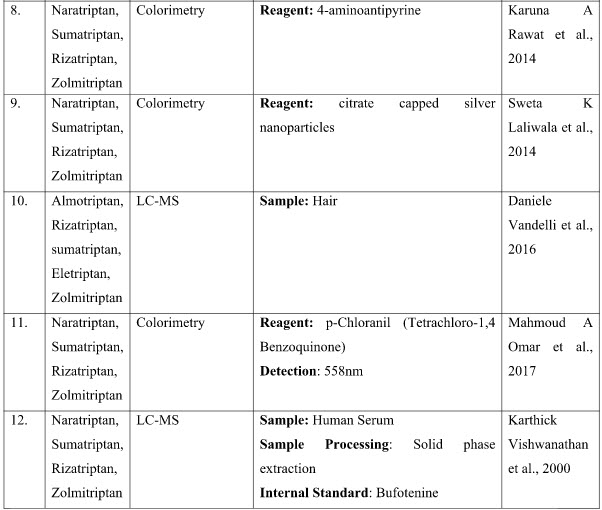
DISCUSSION
The presented exhaustive review covers the analytical methods for the determination of Rizatriptan and its combination in pharmaceutical and biological samples like serum and plasma. Various spectrophotometric and chromatographic conditions are presented in table.
CONCLUSION
The presented information is useful for the future study for researcher involved in qualitative and quantitative analysis of rizatriptan in single and in combination.
REFERENCES
1. Acharjya Sasmita Kumari, Sahoo Subhasish, Dash Kiran Kaushik, Annapurna MM. UV-spectroscopic methods for estimation of Rizatriptan benzoate in pharmaceutical Preparations. International Journal of Chemtech Research. 2010; 2(1): 653-659.
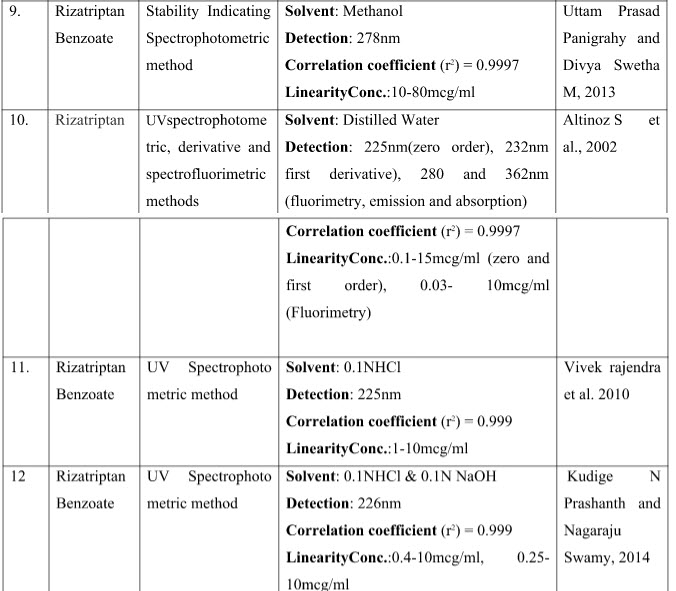
2. Altinoz S, Ucar G, Yıldız E. Determination of Rizatriptan in its tablet dosage forms by UVspectrophotometric and spectrofluorimetric methods. Analytical Letters . 2002; 35(15): 271-2485.
3. Antonucci V, Wright L, Toma P. The Reversed-Phase Liquid Chromatographic Behavior of the New 5-HT1D Receptor agonist Rizatriptan Benzoate and Its potential Process Impurities. Journal of Liquid Chromatography & Related Technologies. 1998; 21(11): 1649-1670.
4. Avula Prameela Rani, Telu Visalakshi, Namasani Santhosh Kumar, Chandra Bala Sekaran. Determination of Rizatriptan Benzoate in Bulk and Tablets by Spectrophotometry. International journal of pharmaceutical and chemical sciences. 2012; 1(4): 1408-1416.
5. Bafna SR, Tarkase KN, Salve MT. Estimation of Rizatriptan benzoate in bulk and in commercial dosage form by UV-spectrophotometry. Int. J. Drug Res. Tech. 2013; 3 (3): 49-52.
6. Barbara G. wells, Joseph T.Dipiro, Terry L.Schwinghammer, Cindy W. Hamilton. Pharmacotherapy handbook. 6th ed. India: Mc.GrawHill; 2005.
7. Barrish A, Olah TV, Gatto GJ, Dobrinska MR, Gilbert JD. The Use of Stable Isotope Labeling and Liquid Chromatography/Tandem Mass Spectrometry Techniques to Study the Pharmacokinetics and Bioavailability of the Antimigraine Drug, MK‐0462 (Rizatriptan) in Dogs. Rapid Communications in Mass Spectrometry. 1996; 10(9): 1033-1037.
8. Bhagawati ST, Reddy MS, Avadani K, Muddukrishna BS, Dengale SJ, Bhat K. Development and validation of reversed-phase high-performance liquid chromatography method for estimation of rizatriptan benzoate in oral strip formulations. J Basic Clin Pharma. 2015; 6(1):7-11.
9. Brain K. Alldredge, Robin L. Corelli, Michael E. Ernst B. Joseph Guglielomo, Pamala A.Jacobson, Wayne A. Kradjan, Bradley R. Williams. Koda-Kimble & Young Applied Therapeutics: The clinical use of drugs. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
10. Cafer Saka. Review of Analytical Methods for Identification and Determination of Triptans. Critical Reviews in Analytical Chemistry. 2009; 39(1): 32-42.
11. Chandra shekhar K Gadewar, Yogendra kumar Sahu, Chandewar AV, Pankaj Baghel, Devendra Kushwaha. Stability indicating method development and validation of assay method for the estimation of rizatriptan benzoate in tablet. Arabian Journal of Chemistry. 2013; 07(036): 1-6.
12. Chen Jun, Gao Xiao-Ling, Jiang Wen-Ming, Jiang Xin-Guo. Determination Of Rizatriptan Benzoate And Its Related Substances By HPLC. Chinese Journal of Pharmaceuticals. 2004-10.
13. Clive Page, Michael Curtis, Michael Walker, Brain Hoffmann. Integrated Pharmacology: Drugs and the Nervous system. 3rd ed. India: Elsevier; 2009.
14. Daniele Vandelli, Federica Palazzoli, Patrizia Verri, Cecilia Rustichelli, Filippo Marchesi, Anna Ferrari, Carlo Baraldi, Enrico Giuliani, Manuela Licata, Enrico Silingardi. Development and validation of a liquid chromatography-tandem mass spectrometric assay for quantitative analyses of triptans in hair. Journal of Chromatography B. 2016; 1017: 136-144.
15. David E. Golan, Armen H. Tashjian, Ehrin J. Armstrong, April W. Armstrong. Principles of Pharmacology: The Pathophysiologic basis of Drug Therapy. 3rd ed. philadelphia: Lippincott Wilkins & Williams; 2012.
16. Dev prakash, Senthilkumar G P, Prithviraj S Yadav, Mani T Tamizh. Determination of Rizatriptan in bulk and its tablet dosage forms by UV Spectroscopic method. International Journal of Pharmaceutical Sciences and Research. 2011; 2(8): 2041-2044.
17. Devprakash, Sumalatha BV, Suhas Gurav, Prithviraj S Yadav, Senthilkumar GP. Estimation of Rizatriptan benzoate by RP-HPLC method in bulk and dosage form. Journal of Pharmacy Research. 2012; 5(1): 120-123.
18. Devprakash Dahiya, Prithviraj Yadav. Determination of Rizatriptan in bulk and its tablet dosage forms by HPTLC method. Journal of Pharmacy Research. 2011; 4(6): 1788-1790.
19. Dinakaran Sathis Kumar, Anusha Pothula, Durganaga Prashanthi Botla, Ahareesh Kumar Pasinibilli, Aavasarala Harani, Bravishankar K. Spectrophotometric method development and validation for naproxen and rizatriptan in bulk and tablet dosage form using absorption ratio method. Pharmanest. 2013; 4(6): 1314-1321.
20. Dinesh S Patel, Naveen Sharma, Mukesh C Patel, Bhavin N Patel, Pranav S Shrivastav, Mallika Sanyal. Application of a Reliable LC-MS/MSMethod for Determination of Rizatriptan in Healthy Subject Samples: Demonstration of Assay Reproducibility by Incurred Sample Reanalysis. International Scholarly Research Network Chromatography.2012; 1-10.
21. Effat Souri, Abbas Kaboodari, Noushin Adib, Massoud Amanlou. A New extractive spectrophotometric method for determination of rizatriptan dosage forms using bromocresol green. DARU Journal of Pharmaceutical Sciences. 2013; 21(12): 1-6.
22. Ganesh Sarowar, Padmanabh Deshpande, Yogesh Gandhi, Janvi Bhatt. Development and Validation of Stability-indicating HPTLC Method for Determination of Rizatriptan as Bulk Drug and in Tablet Dosage Form. J. Chem. Pharm. Res. 2016; 8(4): 1246-1252.
23. Haarika B, Prabhakar Reddy V. A RP-HPLC method development for the estimation of rizatriptan benzoate from formulated fast disintegrating sublingual tablets. International journal of Pharmaceutical Sciences and Research. 2014; 5(9): 3784-3789.
24. Hammad MA, Omar MA, Eltoukhi WE. Validation of rapid and sensitive spectrofluorimetric assay for determination of four triptans in pure and dosage Forms; Application to human plasma and content uniformity testing. Pharm Anal Acta. 2016; 7(7): 1-8.
25. Ishan K Chinnapurkar, Manjusha N Dole and Sanjay D Sawant. Development and validation of analytical methods for estimation of rizatriptan benzoate in bulk and tablet dosage forms by UV spectroscopy. Der Pharmacia Lettre. 2015; 7(3):312-319.
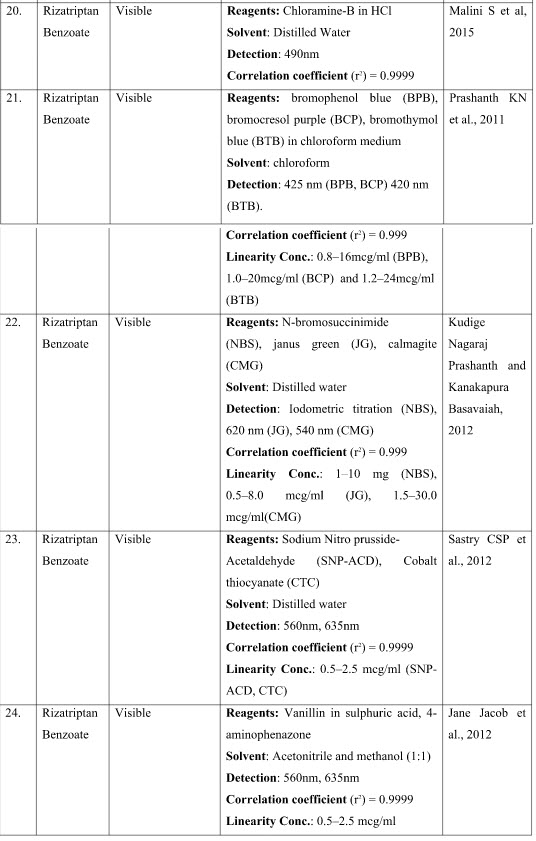
26. Jane Jacob, Devin M Dadhaniya, Patel Vikas, Jani Vishal M, Amit Bangad. Estimation of Rizatriptan in bulk and pharmaceutical formulation. Asian J. Research Chem. 2012; 5(9): 1104-1107.
27. Ji‐fen Guo, Ai‐jun Zhang,Ling Zhao, Xiao‐hong Sun, Yi‐min Zhao, Hong‐zhi Gao, Ze‐yuan Liu, Shan‐yi Qiao. Determination of rizatriptan in human plasma by liquid chromatographic–eletrospray tandem mass spectrometry: application to a pharmacokinetic study. Biomedical Chromatography. 2006; 20(1): 61-66.
28. Jitendra kumar P, Mallikharjuna Rao P, Desi reddy RB, Sandhaya reddy D, Koushik P, Sai kishore V. Development and validation of RP-HPLC method for quantitative analysis of Rizatriptan benzoate in pure and Pharmaceutical formulations. Journal of Pharmacy Research. 2014;8(1):87-90.
29. Jocic B, Zecevic M, Zivanovic Lj, Licanski A. A Chemometrical Approach to Optimization and Validation of an HPLC Assay for Rizatriptan and its Impurities in Tablets. Analytical Letters. 2007; 40(12): 2301-2316.
30. Joseph SunderRaj T, Bharathi Ch, SaravanaKumar M, Joseph Prabahar, Naveen Kumar P, Hemant Kumar Sharma, Kalpesh Parikh. Identification, isolation and characterization of process-related impurities in Rizatriptan benzoate. Journal of Pharmaceutical and Biomedical Analysis. 2009; 49(1): 156-162.
31. Jun Chen, Xinguo Jiang, Wenming Jiang, Ni Mei, Xiaoling Gao, Qizhi Zhang. Liquid chromatographic method for the determination of rizatriptan in human plasma. Journal of Chromatography B. 2004; 805(1): 169-173.
32. Kannappan N, Madhukar A, Ganesh A, Naveen Kumar CH, Mannavalan R. International Journal of Pharm Tech Research. Analytical method development and validation of Rizatriptan benzoate tablets by RP-LC. 2009; 1(4): 1704-1708.
33. Karuna A Rawat, Kiran R Surati, Suresh Kumar Kailasa. One-pot synthesis of gold nanoparticles by using 4-aminoantipyrine as a novel reducing and capping agent for simultaneous colorimetric sensing of four triptan-family drugs. Analytical Methods. 2014; 15.
34. Karthick Vishwanathan, Michael G Bartlett, James T Stewart. Determination of antimigraine compounds rizatriptan, zolmitriptan, naratriptan and sumatriptan in human serum by liquid chromatography/electrospray tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2000; 14(3): 168-172.
35. Kempwade Amolkumar, Taranalli Ashok, Jadhav Kiran. Development and validation of UV Spectrophotometric method to study stress degradation behaviour of Rizatriptan Benzoate. Spectroscopy and Spectral Analysis. 2015; 35(1): 137-140.
36. Koti Reddy Y, Subba Reddy GV, Jaya Veera KN, Kishore Kumar Hotha. UPLC method for the determination of rizatriptan Benzoate and its related impurities. International Journal of Analytical and Bioanalytical Chemistry. 2012; 2(4): 228-234.
37. Kudige N Prashanth, Nagaraju Swamy. Simple and sensitive UV-spectrophotmetric determination of rizatriptan benzoate in pharmaceuticals and its stress studies. Inventi:ppaqa. 2014.
38. Kudige N Prashanth, Kanakapura Basavaiah. Utility of p-Chloranilic Acid and 2,3-Dichloro-5,6-dicyano-p-benzoquinone for the Spectrophotometric Determination of Rizatriptan Benzoate. Analytical Chemistry. 2012;vol 2012. 1-12.
39. Kudige Nagaraj Prashanth, Kanakapura Basavaiah. Sensitive and selective methods for the determination of rizatriptan benzoate in pharmaceuticals using N-bromosuccinimide and two dyes. Journal of Saudi Chemical Society. 2012; 19(2015): 233–242.
40. Mahmoud A Omar, Mohamed A Hammad, Walid E Eltoukhi. Spectrophotometric Determination of Certain Antimigraine Drugs in Pharmaceutical Formulations Using p-Chloranil Reagent; Application to Content Uniformity Testing. Analytical Chemistry Letters. 2017; 7(5): 611-622.
41. Malini S, Kalyan Raj, Veena MA, Nanda N. Kinetic Spectrophotmetric Method for Quantitation of Rizatriptan Benzoate in Pharmaceutical Formulations. International Research Journal of Pure & Applied Chemistry. 2015; 9(1): 1-11.
42. Mallikarjuna Rao B, Sivaiah Sangaraju, Srinivasu MK, Madhavan P, Lalitha Devi M, Rajendra Kumar P, Chandrasekhar KB, Arpitha Ch, Satya Balaji T. Development and validation of a specific stability indicating high performance liquid chromatographic method for rizatriptan benzoate. Journal of Pharmaceutical and Biomedical Analysis. 2006; 41(4): 1146-1151.
43. Mathrusri Annapurna M, Sravya S, Vineesha Ch. New derivative spectrophotometric methods for the determination of rizatriptan benzoate in pharmaceutical dosage forms. International Journal of Pharmaceutical Sciences Review and Research. 2012; 13(2): 133-136.
44. Mira Zecevic, Biljana Jocic, Ljiljana Zivanovic, Ana Protic. Application of Multicriteria Methodology in the Development of Improved RP-LC-DAD for Determination of Rizatriptan and Its Degradation Products. Chromatographia. 2008; 68(11): 911–918.
45. Mohammad Soleimani, Mazaher Ahmadi, Tayyebeh Madrakian, Abbas Afkhami. Magnetic solid phase extraction of rizatriptan in human urine samples prior to its spectrofluorimetric determination. Sensors and Actuators B. 2017; 254 (2018): 1225–1233.
46. Nagappa L.Teradal, Prashanth S.Narayan, Ashis K.Satpati, Jaldappagari Seetharamappa. Fabrication of electrochemical sensor based on green reduction of graphene oxide for an antimigraine drug, rizatriptan benzoate. Sensors and Actuators B: Chemical. 2014; 196: 596-603.
47. Narasimha Rao K, Devanna N, Suresh Reddy KVN. Trace Level Determination of Three Genotoxic Impurities in Drug Samples of Rizatriptan Benzoate by Liquid Chromatography- Tandem Mass Spectrometry. Analytical Chemistry Letters. 2017; 7(2): 248-260.
48. Prasad E Funde , Chandramohan Nibe. A precise analytical method for determination of percent assay in rizatriptan benzoate tablets by RP-HPLC. Applied Research and Development Institute Journal. 2013; 8(4): 20-24.
49. Prashanth KN, Basavaiah K, Vinay KB. Sensitive and selective spectrophotometric assay of rizatriptan benzoate in pharmaceuticals using three sulphonphthalein dyes. Arabian Journal of Chemistry. 2011; 9(2016): 971- 980.
50. Pritam S Jain, Harshal P Chaudhari, Pankaj R Bari, Sanjay J Surana.Area under Curve Method Development and Validation of Rizatriptan Benzoate. International Journal of Pharmaceutical Chemistry and Analysis. 2016; 3(1): 13-17.
51. Pritam S Jain, Harshal P Chaudhari, Sanjay J Surana. Development and Validation of Stability Indicating HPTLC Method for Estimation of Rizatriptan Benzoate in Bulk and Tablet Dosage Form. International Journal of Pharmaceutical Chemistry and Analysis. 2016;3(2):73-79.
52. Punna Venkateshwarlu, Srikanth Gajam. A Validated and simplified RP-HPLC of Rizatriptan benzoate from bulk drugs. Journal of pharmaceutical and biomedical sciences. 2011; 8(08): 1-4.
53. Qin Yong-ping, Zou Yuan-gao, Liang Mao-zhi, Yu Qin. Determination of rizatriptan in human plasma by RP-HPLC with fluorescence detection. Chinese Journal of Pharmaceutical Analysis 2006-01.
54. Rajeev Jain, Tiwari DC, Preeti Pandey. Highly Selective and Sensitive Graphene Based Electrochemical Sensor for Quantification of Receptor Agonist Rizatriptan. Electro Analysis. 2013; 25(6): 1363-1367.
55. Ramakotaiah Mogili, Kanchanamala Kanala, Balasekhara R Challa, Babu R Chandu, Chandrasekhar K Bannoth. Determination of Rizatriptan in Human Plasma by Liquid Chromatography Stable Isotope Dilution Electrospray MS–MS for Application in Bioequivalence Study. Chromatographia.2011; 744(585).
56. Raman NVVSS, Prasad AVSS, Ratnakar Reddy K, Ramakrishna K. Determination of 1-Bromo-3-Chloropropane,1- (4-Nitrobenzyl)- 1H- 1,2,4-Triazole and 1- (Bromo methyl)-4-Nitrobenzene in Rizatriptan Benzoate. Chromatographia. 2017; 80(3): 447–452.
57. Ramzia I El-Bagary, Nashwah G Mohammed, Heba A Nasr. Two Chromatographic Methods for the Determination of Some Antimigraine Drugs. Analytical Chemistry Insights. 2012:7; 13–21.
58. Rang H P, Dale M M, Ritter J M, Flower R J, Henderson G. Rang and Dales Pharmacology. 7th ed. Spain: Churchill Livingstone; 2012.
59. Sachin S Jagtap, Gopu CL, Kakasabeb R Mahadik, Mahadev V Mahadik. Stability indicating reversed-phase high-performance liquid chromatographic method for the determination of rizatriptan benzoate in bulk powder and in pharmaceutical formulations. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2010; 1(2): 385-395.
60. Sai Krishna U, Prasad Rao M, Narasimha Rao D, Sivashankar R Beeravalli. Analytical method development and validation for the estimation of rizatriptan benzoate in pharmaceutical formulation. International Journal of Universal Pharmacy and Bio Sciences. 2013; 2(5): 520-530.
61. Sanmukha kumar JV. Validation of analytical procedures for determination of rizatriptan benzoate. The pharma research. 2010; 4(1).
62. Sastry CSP, Viplava Prasad U, Reddy TS, Acharyulu MLN. Determination of Rizatriptan by Visible Spectrophotometry. Asian J. Research Chem. 2012; 5(12): 1477-1499.
63. Sathis Kumar Dinakaran. Development and validation of RP-HPLC for simultaneous estimation of Rizatriptan and Naproxen in bulk and tablet dosage form. International Journal of Pharmaceutical Research and Development. 2014; 6(1): 79-86.
64. Sethy Prasanta, Mohanty Smita Padma. Estimation of Rizatriptan Benzoate Tablet by Using UV Spectophotometric Methods. Int. J. Pharm. Sci. Rev. Res. 2013; 19(2): 97-100.
65. Shankarananth Velusamy, Venkata Muralidhar Masimukku, Salini Chereddy, Jeevan Kumar Jadapalli, Keerthisikha Palur, Sreenivasa Charan Archakam, Rajasekhar Komarla Kumarachari. Bioanalytical method development and validation of rizatriptan in human plasma using LC-MS/MS method. International journal of chemical and analytical science. 2013; 4: 108-114.
66. Sharma S, Sharma MC. Densitometric Application for Rizatriptan Benzoate in Bulk and Dosage Forms. World Journal of Chemistry. 2011; 6 (1): 49-52.
67. Sirisha V, Sreedhar C, Sreenivasa Rao T. Analytical Method Development and Validation for Quantitative Estimation of Rizatriptan Benzoate. 2013; 2(1): 1-4. doi:10.4172/scientificreports.620.
68. Srinivasula Reddy Maddula, Manoj Kharkar, Kushal Manudhane, Sandeep Kale, Abijar Bhori, Arvind Lali, Dubey PK, Janardana Sarma KR, Apurba Bhattacharya, Rakeshwar Bandichhor. Preparative Chromatography Technique in the Removal of Isostructural Genotoxic Impurity in Rizatriptan: Use of Physicochemical Descriptors of Solute and Adsorbent. Org. Process Res. Dev. 2009; 13 (4): 683–689.
69. Sundar BS, Suneetha A, Rajeswari NR, Prasanna VL. Validation of RP-HPLC analytical method for quantitation of Rizatriptan benzoate in tablet dosage form. Indian drugs. 2009; 46(5): 422-425.
70. Suneetha A, Syama Sundar B. New simple UV spectrophotometric method for estimation of rizatriptan benzoate in bulk and Pharmaceutical dosage forms. J. Pharma. Tech, and Res. 2009; 1(1): 38-40.
71. Sunil S Khanchandani, Upendra C Galgatte, Praveen D Chaudhari. Development And Validation Of UV-Visible Spectroscopic Method For Estimation Of Rizatriptan Benzoate In Bulk And Tablet Dosage Form. Asian J Pharm Clin Res. 2013; 6(4), 113-116.
72. Sweta K Laliwala, Vaibhav kumar N Mehta, Jigneshkumar V Rohit, Suresh Kumar Kailasa. Citrate-modified silver nanoparticles as a colorimetric probe for simultaneous detection of four triptan-family drugs. Sensors and Actuators B: Chemical. 2014; 197: 254-263.
73. Syama Sundar B, Suneetha A. Development and Validation of HPTLC Method for the Estimation of Rizatriptan Benzoate in Bulk and Tablets. Indian Journal of Pharmaceutical Sciences. 2010; 798-801.
74. Tayyebeh Madrakian, Somayeh Maleki, Mozhgan Heidari, Abbas Afkhami. An electrochemical sensor for rizatriptan benzoate determination using Fe3O4 nanoparticle/multiwall carbon nanotube-modified glassy carbon electrode in real samples. Materials Science and Engineering: C. 2016; 63: 637-643.
75. Tripathi K D. Essentialities of Medical Pharmacology 6th ed. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2006.
76. Uttam Prasad Panigrahy, Divya Swetha M. Development and validation of stability indicating spectro-photometric method for the estimation of Rizatriptan benzoate in bulk and pharmaceutical dosage form. International Journal of Pharmaceutical Sciences and Research. 2013; 4(10): 4046-4050.
77. Vishal P Awari, Subramania Nainar Meyyanathan, Yamjala Karthik, Natarajan Jawahar. HPLC Method Development and Validation of Rizatriptan in Rabbit Plasma. J. Pharm. Sci. & Res. 2014; 6(1): 24 – 26.
78. Vivek rajendra, amol khedkar, alpana kulkarni, mohd. Hassan g dehghan, maria Saifee, Swaroop Lahoti. Spectrophotometric Estimation of Rizatriptan Benzoate. Asian J. Research Chem. 2010; 3(1): 175-177.
79. Vivek Sagar P, Dhiraj Kumar, Suddhasattya Dey, Himansu Bhusan Samal. Simultaneous estimation of rizatriptan, sumatriptan and zolmitriptan by RP-HPLC method in bulk. Journal of Pharmacy Research. 2010; 3(12): 2930-2933.
80. Waldman, Terzic Pharmacology and Therapeutics: Principles to practice. ed. Philadelphia: Saunders Elsevier; 2009.
81. www.drugs.com/monograph/rizatriptan-benzoate.html
82. www.drugfuture.com/Pharmacopoeia/USP35/data/v35300/usp35nf30s0_alpha-2-31.html
83. www.medicinenet.com/rizatriptan_tablet-oral/page5.htm
84. www.nlm.nih.gov/medlineplus/druginfo/meds/a601109.html#
85. www.wikipedia.com
86. Yi Chen, Haijun Miao, Mei Lin, Guorong Fan, Zhanying Hong, Huiling Wu, Yutian Wu. Development and validation of a selective and robust LC–MS/MS method for high-throughput quantifying rizatriptan in small plasma samples: Application to a clinical pharmacokinetic study. Journal of Chromatography B. 2006; 844: 268–277.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









