{ DOWNLOAD AS PDF }
ABOUT AUTHORS
1Jyoti Yadav , 2Amit Sharma*
1Maharishi Dayanad University Rohatak
2 NIMS University, Jaipur
*amitsharma84945@gmail.com
ABSTRACT
This review article is intended to highlight the analytical methods of aspirin, clopidogrel and rosuvastatin in individual as well as combined pharmaceutical dosage form. Aspirin, clopidogrel and rosuvastatin play an important role in the various cardiovascular diseases. Aspirin and clopidogrel are the antiplatelet whereas Rosuvastatin is antilipemic agent which are used in the treatment of various cardiovascular diseases, cerebrovascular and peripheral vascular diseases. Now these days these drugs are easily available in the market in their individual form as well as in their combined dosage form. Aspirin, clopidogrel and rosuvastatin are official in the pharmacopoeias .Various analytical methods have been reported for the estimation of these drugs in their individual form as well as in their combined dosage form.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2518
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 9 Received On: 22/04/2017; Accepted On: 14/05/2017; Published On: 01/09/2017 How to cite this article: Yadav J, Sharma A;A Review on Analytical Methods for Estimation of Aspirin, Clopidogrel Bisulphate and Rosuvastatin Calcium in Pharmaceutical Dosage Form; PharmaTutor; 2017; 5(9);35-46 |
Introduction to Analytical Method
There are various analytical methods are used nowthese days for the estimation .Various analytical methods like potentiometer, HPLC ,aqueous and non-aqueous titrations are used in the field of analysis .Aqueous and non-aqueous titrations are also used in the field of analysis .But now these days HPLC plays an important role in the field of analysis for the quantitative determination.
HPLC is referred as high pressure liquid chromatography which is a separation technique based on the solid stationary phase and liquid mobile phase [1].Chromatography is mass transfer process involve adsorption. The active component of the column is adsorbent which is granular material of solid particles(silica, polymers). The principle of separation in the normal phase mode and reverse phase mode is adsorption in which the substances travel /separate according to their relative affinities. Now these days HPLC plays an important role in the field of pharmaceutical analysis for the separation of various substances from the mixture of substances [2].
Introduction to Drug Profile
Aspirin
Aspirin is known as acetylsalicylic acid which is still the most commonly used NSAID to treat pain and inflammation [3].Aspirin is 2-acetyloxy benzoic which is COX inhibitor. Aspirin is white crystalline powder[4] which is freely soluble in chloroform and in ether, slightly soluble in water with having molecular formula C9H8O 4 and molecular weight 180.2g/mol.
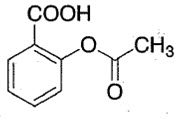
Chemical Structure of Aspirin
Pharmacological action [5]
The analgesic, antipyretic, and anti-inflammatory effects of acetylsalicylic acid are due to actions by both the acetyl and the salicylate portions of the intact molecule as well as by the active salicylate metabolite. Acetylsalicylic acid directly and irreversibly inhibits the activity of both types of cyclooxygenase (COX-1 and COX-2) to decrease the formation of precursors of prostaglandins and thromboxane’s from arachidonic acid. This makes acetylsalicylic acid different from other NSAIDS (such as diclofenac and ibuprofen) which are reversible inhibitors.
Summary of Analytical Methods for Aspirin
Official Methods for Aspirin6-8
|
SR. No. |
Official in |
METHOD |
BRIEF INTRODUCTION |
REF. NO |
|
1 |
IP-2010 (Aspirin tablet) |
Potentiometric Titration |
Titrate:- Tablet Powder Equivalent to 0.5 gm. Aspirin in 30ml of the 0.5M Sodium Hydroxide Titrant:- 0.5 M HCl
1ml of 0.5M NaOH is Equivalent to .0.04504 gm. of Aspirin |
6 |
|
2 |
BP-2009 |
Potentiometric Titration |
Titrate:- 1gm Aspirin in 10ml Ethanol, Add50ml of the 0.5M Sodium Hydroxide Titrant:- 0.5 M HCl
1ml of 0.5M NaOH is Equivalent to .0.04504 gm. of Aspirin |
7 |
|
3 |
USP30-NF25 (Aspirin Tablet) |
Liquid Chromatography |
Mobile phase:- Water (pH 3.4): Acetonitrile (85:15) Column:- Packing L1, (300 mm × 4.0 mm) Flow rate:- 2 ml/min Wavelength:- 285 nm. |
8 |
Reported Methods for Aspirin14-26
|
SR. No. |
DRUGS |
METHOD |
BRIEF INTRODUCTION |
REF. NO |
|
1 |
Aspirin |
RP-HPLC |
Mobile phase:- Sodium Perchlorate Buffer, pH (2.5): Acetonitrile: Isopropyl alcohol (85:14:1) Column:- C18, (100 mm × 4.6 mm, 5µ,) Flow rate:- 1.5 ml/min Wavelength:- 275 nm. |
9 |
|
2 |
Aspirin and Metoprolol |
RP-HPLC |
Mobile phase:- Phosphate Buffer, (pH 4.6): Methanol (20:80) Column:- Phenomenex Luna C18, (250 mm × 4.6 mm, 5µ,) Flow rate:- 0.8 ml/min Wavelength:- 230 nm. |
10 |
|
3 |
Aspirin, Ramipril, Hydrochlorothiazide, Simvastatin And Atenolol |
RP-HPLC |
Mobile phase:- Methanol: Water (95:5) Column:- Hypersil Gold C18, (250 mm × 4.6 mm, 5µ,) Flowrate:- 1 ml/min Wavelength:- 230 nm. |
11 |
|
4 |
Aspirin and Prasugrel |
RP-HPLC |
Mobile phase:- Acetonitrile: Acetate Buffer (75:25) Column:- Luna C18 (150 mm × 4.6 mm, 5µ,) Flow rate:- 0 .6 ml/min Wavelength:- 245 nm |
12 |
|
5 |
Aspirin and Prasugrel |
Stability indicating RP-HPLC |
Mobile phase:- Acetonitrile: Methanol: Water pH (3) (30:10:60) Column:- Kromasil-100 C18 (150 mm × 4.6 mm, 5µ,) Flow rate:- 1 ml/min Wavelength:- 245 nm |
13 |
|
6 |
Aspirin and Salicylic acid |
RP-HPLC |
Mobile phase:- Acetonitrile: Trifluoroacetic acid 0.05% (30:70) Column:- Waters C18 (250 mm × 4.6 mm, 5µ,) Flow rate:- 1.0 ml/min Wavelength:- 230 nm |
14 |
|
7 |
Amlodipine Besylate, Atenolol and Aspirin |
RP-HPLC |
Mobile phase:- Methanol: Phosphate Buffer (pH 7.0) (70:30) Column:- BDS C18 (250 mm × 4.6 mm, 5µ,) Flow rate:- 1.0 ml/min Wavelength:- 235 nm |
15 |
|
8 |
Aspirin And Aspirin Derivatives |
RP-HPLC |
Mobile phase:- Acetonitrile: Water (60:40) Column:- Kromasil C18 (180 mm × 4.6 mm, 5µ,) Flowrate:- 1.0 ml/min Wavelength:- 277 nm |
16 |
|
9 |
Aspirin, Caffeine and Orphenadrine citrate |
RP-HPLC |
Mobile phase:- Methanol: Phosphate Buffer, pH3 (65:35) Column:- Acclaim C18 (250 mm × 4.6 mm, 5µ,) Flowrate:- 1.0 ml/min Wavelength:- 215 nm |
17 |
|
10 |
Aspirin and Dipyridamole |
RP-HPLC |
Mobile phase:- 0.1 % Phosphoric acid: Acetonitrile (75:25) Column:- RP C18 (50 mm × 4.6 mm, 3.5µ,) Flow rate:- 1.0 ml/min Wavelength:- 227 nm |
18 |
|
11 |
Aspirin and Esomeprazole Magnesium |
RP-HPLC |
Mobile phase:- Acetonitrile: Methanol: Phosphate Buffer, pH 3.0 (25:25:50) Column:- ODS BP C18 (200 mm × 4.6 mm, 5µ,) Flow rate:- 1.0 ml/min Wavelength:- 230 nm |
19 |
|
12 |
Ramipril, Aspirin and Simvastatin |
RP-HPLC |
Mobile phase:- Acetonitrile: Methanol: 0.5% phosphoric acid (10:70:20) Column:- ODS BP C18 (200 mm × 4.6 mm, 5µ,) Flow rate:- 1.0 ml/min Wavelength:- 226 nm |
20 |
|
13 |
Aspirin, Salicylic Acid, and Caffeine |
RP-HPLC |
Mobile phase:- Water: Methanol: Acetic acid (69:28:3) Column:- Hypersil C18 (150 mm × 4.6 mm, 5µ,) Flow rate:- 1.0 ml/min Wavelength:- 275 nm |
21 |
Clopidogrel Bisulphate[22-23]
Clopidogrel Bisulphate is an antiplatelet agent which is used to inhibit the aggregation of platelets which inhibits the blood clots .The drug is Methyl (+)-(S)-α-(2-chlorophenyl)-6,7dihydrothieno [3,2-c] pyridine-5(4H) acetate sulfate. Clopidogrel bisulphate is insoluble in water.Clopidogrel Bisulphate is an irreversible inhibitor of P2Y12. The molecular formula of clopidogrel bisulphate is C16H16ClNO2S.H2SO4 and the molecular mass is 419.03 g/mol.
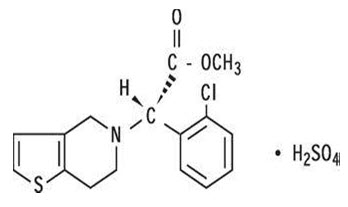
Pharmacological Action [23]
Clopidogrel is an anti-platelet agent which acts by direct inhibition of ADP. The anti –aggregating activity of the clopidogrel bisulphate is due to the biotransformation of the drug to 2-oxo-clopidogrel by enzyme P450-1A.Clopidogrel Bisulphate is mostlyused in the myocardial infarction, stroke and peripheral artery disease
Summary of Analytical Methods for Clopidogrel Bisulphate
Official Methods for Clopidogrel Bisulphate[24-25]
|
SR. NO |
OFFICIAL IN |
METHOD |
DESCRIPTION |
REF. NO |
|
1 |
IP 2010 (Clopidogrel Tablet) |
Chiral Chromatography |
Mobile phase:- Phosphate Buffer: Acetonitrile (75:25) Column:- Chiral Recognition Protein (15 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 220 nm |
24 |
|
2 |
USP30-NF25 ( Clopidogrel Tablet) |
Chiral Chromatography |
Mobile phase:- Phosphate Buffer: Acetonitrile (75:25) Column:- Packing L57 (15 cm X 4.6 mm) Flow Rate:- 1.0 ml/min Wavelength:- 220 nm |
25 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Reported Methods for Clopidogrel Bisulphate [26-39]
|
SR NO. |
DRUGS |
METHOD |
BRIEF INTRODUCTION |
REF. NO. |
|
1 |
Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 2.8: Acetonitrile (35:65) Column:- Develosil ODS (15 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 225 nm |
26 |
|
2 |
Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 4.0: Acetonitrile (32:68) Column:- Hypersil BDS C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 220 nm |
27 |
|
3 |
Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Phosphate Buffer: Acetonitrile, Methanol (10:80:10) Column:- Knauer C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 0.9 ml/min Wavelength:- 240 nm |
28 |
|
4 |
Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 3.0: Acetonitrile (40:60) Column:- C18 (15 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 224 nm |
29 |
|
5 |
Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- 0.1% Trifluoroacetic acid: Acetonitrile (30:70) Column:- Inertsil ODS C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 220 nm |
30 |
|
6 |
Clopidogrel |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 3.0: Acetonitrile (75:25) Column:- ODS C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 247 nm |
31 |
|
7 |
Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 8.0: Acetonitrile (30:70) Column:- Nova pack C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 0.8 ml/min Wavelength:- 210 nm |
32 |
|
8 |
Clopidogrel Bisulphate |
Stability Indicating RP-HPLC |
Mobile phase:- Tetrabutyl ammonium Hydrogen Sulfate Buffer: Acetonitrile (70:30) Column:- Symmetry C8 (15 cm X 3.9 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 225 nm |
33 |
|
9 |
Clopidogrel Bisulphate |
Stability Indicating RP-HPLC |
Mobile phase:- Phospahte Buffer, pH 4.0: Acetonitrile (80:20) Column:- C18 (15 cm X 4.6 mm),5 µm Flow Rate:- 0.5 ml/min Wavelength:- 235 nm |
34 |
|
10 |
Clopidogrel Bisulphate and Atorvastatin Calcium |
RP-HPLC |
Mobile phase:- Acetonitrile: Water (65:35) Column:- Sphere-100 C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 0.5 ml/min Wavelength:- 227 nm |
35 |
|
11 |
Clopidogrel Bisulphate and Atorvastatin Calcium |
RP-HPLC |
Mobile phase:- Solvent A: 0.1% Trifluoro acetic acid in water Solvent B: 0.1% Trifluoro acetic acid in Acetonitrile Column:- X-Bridge C18 (15 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 215 nm |
36 |
|
12 |
Clopidogrel Bisulfate, Its Carboxylic Acid Metabolite, and Atorvastatin |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 2.6: Acetonitrile: Methanol Column:- Hypersil BDS C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 220 nm |
37 |
|
13 |
Phenytoin Sodium and Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Water, pH 3.0: Acetonitrile (30:70) Column:- Phenomenx Luna C18 (15 cm X 4.6 mm),5 µm Flow Rate:- 0.5 ml/min Wavelength:- 215 nm |
38 |
|
14 |
Clopidogrel And Pioglitazone |
RP-HPLC |
Mobile phase:- Water, pH 4.6: Acetonitrile: Methanol (10:10:80) Column:- C18 (15 cm X 4.6 mm),5 µm Flow Rate:- 1 ml/min Wavelength:- 230 nm |
39 |
Rosuvastatin Calcium[40]
Rosuvastatin calcium is referred as statin which is a cholesterol lowering drug .The IUPAC name of rosuvastatin is [(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl-(methylsulfonyl) amino] pyrimidin-5-yl] (3R, 5S)-3, 5-dihydroxyhept-6-enoicacid][40].The chemical formula of rosuvastatin calcium isC22H27FN3O6S)2.Ca and molecular mass of rosuvastatin calcium is 1001.1 g/mol.
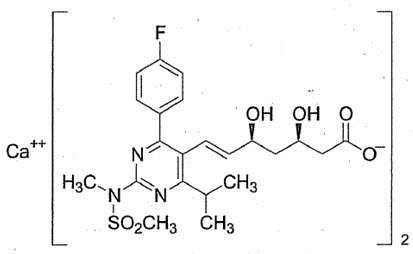
Pharmacological Action[41]
Rosuvastatin is lipid lowering agent which inhibits the HMG-CoAwhich prevents the conversion of 3-hudroxy -3-methylglutaryl-coenzyme-A to melvonate which is precursor of cholesterol [41].Rosuvastatin is also used in the treatment of atherosclerosis, heartattack, stock and peripheral vascular disease.
Summary of Analytical Methods of Rosuvastatin Calcium
Official Methods of Rosuvastatin Calcium[42]
|
SR. NO |
OFFICIAL IN |
METHOD |
DESCRIPTION |
REF. NO |
|
1 |
IP 2010 (Rosuvastatin Tablet) |
RP-HPLC |
Mobile phase:- Acetate Buffer, pH 4.0: Acetonitrile: Tetrahydrofuran (59:36:5) Column:- C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.5 ml/min Wavelength:- 248 nm |
42 |
Reported Methods for Rosuvastatin Calcium [43-57]
|
SR NO. |
DRUGS |
METHOD |
BRIEF INTRODUCTION |
REF. NO. |
|
1 |
Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Water, pH 3.5: Acetonitrile (60:40) Column:- YMC C8 (15 cm X 4.6 mm),5 µm Flow Rate:- 1.5 ml/min Wavelength:- 242 nm |
43 |
|
2 |
Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 3.0: Acetonitrile (50:50) Column:- Thermo Hypersil C18 (10 cm X 4.6 mm),5 µm Flow Rate:- 0.5 ml/min Wavelength:- 243 nm |
44 |
|
3 |
Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 4.5: Acetonitrile: Methanol (50:50) Column:- Luna C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 248 nm |
45 |
|
4 |
Rosuvastatin |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 6.8: Acetonitrile: (60:40) Column:- RP C18 (10 cm X 4.6 mm),3 µm Flow Rate:- 0.6 ml/min Wavelength:- 242 nm |
46 |
|
5 |
Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Acetonitrile: Water (75:25) Column:- Enable C18 (25 cm X 4.6 mm),5 µm Flow Rate:-0.6 ml/min Wavelength:- 252 nm |
47 |
|
6 |
Rosuvastatin Calcium |
Stability indicating RP-HPLC |
Mobile phase:- Solvent-A: Acetonitrile: Water: Methanol: Tetrahydrofuran (10:40:2:5) Solvent-B: Acetonitrile: Methanol: Tetrahydrofuran (50:5:0.5) Column:- Luna C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 2.0 ml/min Wavelength:- 243 nm |
48 |
|
7 |
Rosuvastatin Calcium and Ezetimibe |
RP-HPLC |
Mobile phase:- Acetonitrile: Water (75:25) Column:- Enable C18 (25 cm X 4.6 mm),5 µm Flow Rate:-0.6 ml/min Wavelength:- 252 nm |
49 |
|
8 |
Rosuvastatin and Ezetimibe |
RP-HPLC |
Mobile phase:- Phosphate Buffer: Acetonitrile: Methanol (40:15:45) Column:- Zorbax C18 (15 cm X 4.6 mm),3.5 µm Flow Rate:-1.5 ml/min Wavelength:- 242 nm |
50 |
|
9 |
Rosuvastatin and Ezetimibe |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 8.0: Acetonitrile: Water (50:40:10) Column:- Waters C18 (25 cm X 4.6 mm),3.5 µm Flow Rate:-1.0 ml/min Wavelength:- 230 nm |
51 |
|
10 |
Rosuvastatin and Ezetimibe |
Stability indicating RP-HPLC |
Mobile phase:- Acetate Buffer, pH 6.5: Acetonitrile (55:45) Column:- Sunfire BDS C18 (25 cm X 4.6 mm),3.5 µm Flow Rate:-0.8 ml/min Wavelength:- 230 nm |
52 |
|
11 |
Rosuvastatin and Fenofibrate |
RP-HPLC |
Mobile phase:- Water, pH 2.5: Acetonitrile (30:70) Column:- Inertsil ODS C18 (25 cm X 4.6 mm),3.5 µm Flow Rate:-1.0 ml/min Wavelength:- 248 nm |
53 |
|
12 |
Rosuvastatin Calcium and Fenofibrate |
RP-HPLC |
Mobile phase:- Water: Acetonitrile: Methanol (20:40:40) Column:- Agilent ODS C18 (25 cm X 4.6 mm),3.5 µm Flow Rate:-1.0 ml/min Wavelength:- 252 nm |
54 |
|
13 |
Rosuvastatin Calcium and Fenofibrate |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 5.5: Methanol (25:75) Column:- Phenomenex C18 (25 cm X 4.6 mm),3.5 µm Flow Rate:-1.0 ml/min Wavelength:- 272 nm |
55 |
|
14 |
Rosuvastatin Calcium and Niacin |
RP-HPLC |
Mobile phase:- Phosphate Buffer: Acetonitrile (50:50) Column:- Inertsil ODS C18 (15 cm X 4.6 mm),3.5 µm Flow Rate:-1.0 ml/min Wavelength:- 254 nm |
56 |
|
15 |
Rosuvastatin calcium and Amlodipine besylate |
RP-HPLC |
Mobile phase:- Acetonitrile: Tetrahydrofuran and Water, pH 3.0 (68:12:20) Column:- Qualisil C8 (25 cm X 4.6 mm),3.5 µm Flow Rate:-1.0 ml/min Wavelength:- 251 nm |
57 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
REPORTED METHOD FOR ASPIRIN, CLOPIDOGREL BISULPHATE AND ROSUVASTATIN CALCIUM WITH EACH OTHER 58-67
|
SR NO. |
|
DRUGS |
METHOD |
BRIEF INTRODUCTION |
REF. NO. |
|
1 |
|
Aspirin and Clopidogrel |
RP-HPLC |
Mobile phase:- 3% o-Phosphoric acid: Acetonitrile (65:35) Column:- Phenomenex C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 266 nm |
58 |
|
2 |
|
Aspirin and Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Acetonitrile: Phosphate Buffer, pH 3.0: Methanol (50:30:20) Column:- C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.5 ml/min Wavelength:- 240 nm |
59 |
|
3 |
|
Aspirin and Clopidogrel |
Stability indicating RP-HPLC |
Mobile phase:- Solvent A: Phosphate Buffer, pH 2.3 Solvent B: Methanol: Acetonitrile (50:50) Column:- Phenyl Hexyl (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 220 nm |
60 |
|
4 |
|
Aspirin and Clopidogrel Bisulphate |
Ion pair RP-HPLC |
Mobile phase:- Acetonitrile: Tetrabutylammonium Hydrogen Sulphate Buffer (50:50) Column:- Lichrosphere-100 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 240 nm |
61 |
|
5 |
|
Aspirin, Atorvastatin Calcium and Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Acetonitrile: Phosphate Buffer, pH 3.0 (50:50) Column:- Inertsil ODS (15 cm X 4.6 mm),5 µm Flow Rate:- 1.2 ml/min Wavelength:- 235 nm |
62 |
|
6 |
|
Aspirin, Atorvastatin Calcium and Clopidogrel Bisulphate |
RP-HPLC |
Mobile phase:- Acetonitrile: Water, pH 3.0:Methanol (50:40:10) Column:- Hypersil BDS C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 248 nm |
63 |
|
7 |
|
Aspirin and Rosuvastatin |
RP-HPLC |
Mobile phase:- Methanol: Buffer (45:55) Column:- X-Terra C18 (15 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 215 nm |
64 |
|
8 |
|
Aspirin and Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Water with 0.5% Triethylamine: Acetonitrile (50:50) Column:- Smart C18 (25 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 243 nm |
65 |
|
9 |
|
Aspirin and Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Phosphate Buffer, pH 3.0: Acetonitrile (45:55) Column:- Hyper chrome ODS BP (20 cm X 4.6 mm),5 µm Flow Rate:- 1.0 ml/min Wavelength:- 241 nm |
66 |
|
10 |
|
Clopidogrel Bisulphate and Rosuvastatin Calcium |
RP-HPLC |
Mobile phase:- Perchlorate Buffer, pH 2.5: Acetonitrile (65:35) Column:- Nova pack C18 (10 cm X 3.9 mm),4 µm Flow Rate:- 1.0 ml/min Wavelength:- 242 nm |
67 |
CONCLUSION
Aspirin, clopidogrel bisulphate and rosuvastatin calciumplay an important role in the many cardiovascular diseases, and in various diseases .These drugs are available in the market in many formulations with their different dose .Many methods have been reported for the estimation of these drugs but currently not any method have been reported for the simultaneous estimation of these drugs in their combined dosage form. So there is need to develop a suitable, accurate and validated method for their simultaneous estimation in combined dosage form.
REFERENCE
1. Kazakevich Y and LoBrutto: RP HPLC for pharmaceutical Scientists; A John Wiley and sons, 2007, pp 1-6.
2. Vibha Gupta, Ajay Deep Kumar Jain, N. S. Gill, Kapil Gupta “Development and validation of HPLC method - A review” Int. Res J Pharm. App Sci., 2012; 2(4):17-25.
3. Md. Gousuddin, Pinaki Sengupta, Vijaya Datt Tripathi, Arindam Das “stability indicating RP-HPLC method for simultaneous determination of aspirin and clopidogrel in dosage form”.
4. US. Ramjith, DK. Sunith, Smrithi Radhakrishnan and PA. Sameer “HPLC study of aspirin and aspirin derivatives international journal of research in pharmacy and chemistry” ISSN: 2231-2781.
5. Dr. Vinit Swami, Dr. Vasanthi Swami “Effect of nonsteriodal anti-inflammatory drugs on orthodontic tooth movement – review” IOSR Journal of Pharmacy (e)-ISSN: 2250-3013, (p)-ISSN: 2319-4219.
6. Indian Pharmacopeia-2010, Indian Government Health and Welfare Society, Ghaziabad, pp843.
7. British Pharmacopoeia-2009, Official Monograph of Aspirin, pp1-5.
8. USP30-NF25, Pharmacopeia Forum: Volume No. 29(6) pp 1446.
9. Kumar SS, Jamadar LD, Bhat K, Musmade PB, Vasanthrsju SG, “Analytical Method Development and Validationfor Aspirin” Int. J. Chem. tech. Res., 2010, 2(1), 389-399.
10. Tstvetkova BG, Pancheva IP, Peikov PT, “Development and validation of RP-HPLC method for simultaneousdetermination of metoprolol and aspirin in fixed dose combinations” Der pharma chemical, 2012, 4(4), 1512-1516.
11. Yadav S, Rao JR, “RP-HPLC Method for Simultaneous Estimation Of Aspirin, Ramipril, Hydrochlorothiazide, Simvastatin And Atenolol From PharmaceuticalDosage Form” Int. J. Pharm and pharm. Sci., 2014, 6(9), 443-448.
12. Jain DK, Jain N, Verma J, “RP-HPLC Method for Simultaneous Estimation of Aspirin andPrasugrel in Binary Combination” Int. J. Pharm. Sci. And drug res., 2012, 4(3), 218-221
13. Patel SM, Patel CN, Patel VB, “Stability indicating HPLC Method for Simultaneous Determination of Aspirin and Prasugrel” Int. J. Pharm. Sci., 2013, 75(4), 413-419.
14. Leandro KC, Abrantes MB, “Development of a new analytical method for determination of acetylsalicylic and salicylic acids in tablets by reversed phase liquidchromatography” Braz. J. Pharm.sci., 2009, 45(4), 723-727.
15. Bhusari VK, Dhaneswar SR, “Validated HPLC Method for Simultaneous Quantitation of Amlodipine Besylate, Atenololand Aspirin in Bulk Drug and Formulation” J. Pharm. And biomed sci., 2012, 17(9), 1-6.
16. Ramjith US, Sunith DK, Radhakrishnan S, Sameer PA, “HPLC Study Of Aspirin And Aspirin Derivatives” Int. J. Res. In pharm. And chem.., 2013, 3(1), 1-5.
17. Pai SP, Gaude S, Palekar A, “RP-HPLC Method Development and Validation for Simultaneous Estimation of Aspirin, Caffeine andOrphenadrine citrate in Tablet Formulation” Int. J. Sci. and res., 2013, 5(1), 1170-1173.
18. Prakash K, Kalakunta RR, Sama SR, “Rapid and simultaneous determination of aspirin and dipyridamole in pharmaceutical formulations by reversed-phase high performance liquidchromatography (RP-HPLC) method” African j.pharm and pharmacology, 2011, 5(2), 244-251
19. Patel D, Patel N, Vishy R, Patel V, “Development and Validation of RP-HPLC Method for Simultaneous Estimation of Aspirin and EsomeprazoleMagnesium in Tablet Dosage Form” Hindawi Pub. Corp., 2013, 5
20. Kapugandi AN, Gandhi BM, Raju VB, “Development and Validation of Stability Indicating RP-HPLC Method for Simultaneous Estimation of Ramipril, Aspirin and Simvastatin in Bulk and Pharmaceutical Dosage Form” Asian J. Biomed and pharm sci., 2016, 6(53), 14-20.
21. Sawyer M, Kumar V, “A Rapid High-Performance Liquid Chromatographic Method for the Simultaneous Quantitation of Aspirin,Salicylic Acid, and Caffeine in Effervescent Tablets” J. Chrom. Sci., 2003, 41, 393-397.
22. Bhagat Dimple, Mannur Vinodh, Mastiholimath Vinayak, “Development and Validation of RP-HPLC Method for the Estimation of clopidogrel Bisulphate” Malaysian Journal of Analytical Sciences, Vol. 17 No 3 (2013): 387 – 393.
23. A.Mounika, N.Sriram, “Method Development and Validation of Clopidogrel Bisulphate by Reverse Phase-HPLC in Bulk and Pharmaceutical Dosage Forms” IJPAR |Volume 1 | Issue 1 | Dec – 2012.
24. Indian Pharmacopeia-2010, Indian Government Health and Welfare Society, Ghaziabad, pp 1119-1120
25. USP30-NF25, Pharmacopeia Forum: Volume No. 32(1) pp 74.
26. Sahoo NK, Sahu M, Rao PS, Indira JN, Rani SN, Ghosh GK, “Validation of assay for bulk clopidogrel and for some tablet forms by reverse-phase high-performance liquid chromatography” J. Taib. Uni., 2014, 8, 331-336
27. Bhagat D, Mannur V, Mastiholimath V, “Development and Validation of RP-HPLC Method for The Estimation Of Clopidogrel Bisulphate” Mal. J. Anal. Sci., 2013, 17(3), 387-393
28. Ammar MA, Haider S, Mando H, “Development And Validation Of RP-HPLC Method For Determination Of Clopidogrel In Tablets” Int. J, pharm.sci. rev, res., 2012, 14(2), 1-5
29. Maunika A, Sriram N, “Method Development and Validation of Clopidogrel Bisulphate by Reverse Phase-HPLC in Bulk and Pharmaceutical Dosage Forms” Int. J. Pharm. And anal. Res., 2012, 1(1), 1-7
30. Mayee KK, Rathma T, Prahlad R, “Development and Validation of RP-HPLC Method for the Estimation of Clopidogrel Bisulphate in Tablet Dosage Form” Int. J. Res. In pharm. And nano sci., 2013, 2(3), 293-304
31. Housheh S, Daoud A, Trefi S, Haroun M, “Optimization of RP-HPLC Assay for Pharmaceutical Analysis of Clopdogrel” Int. J. Pharm. Sci. And Nano tech., 2014, 7(1), 2771-2776
32. Dermis S, Aydogan E, “Rapid And Accurate Determination Of Clopidogrel In Tablets By Using Spectrophotometric And Chromatographic Techniques” Common fac. Sci. Uni., 2009, 55(1), 1-16
33. Krishna VS, Kumar DR, Balamurlikrishna K, Rambabu C, “Development and validation of stability indicating RP-HPLC method for the determination of clopidogrel bisulphate in bulk and its dosage forms” Der Pharm. Chem.., 2014, 6(2), 366-374
34. Alarfaz NA, “Stability-indicating liquid chromatography for determination of clopidogrel bisulphate in tablets: Application to content uniformity testing” J. Saudi Chem. Soc., 2012, 16, 23-30
35. Gosavi NP, Patil VV, Patil VR, “Development and Validation of Analytical and Method for the Simultaneous Estimation of Clopidogrel Bisulphate and Atorvastatin Calcium in Bulk and in Tablet” Res. J. Pharm. Chem. and res. Sci., 2012, 3(3), 1065-1071
36. Niharika M, Kumari KS, Rahaman SA, Maheshwari G, “RP-HPLC Method For Simultaneous Estimation of Clopidogrel Bisulphate And Atorvastatin Calcium in a Capsule Dosage Form” Indo ame j. pharm. Res., 2013, 7087-7094
37. Croitoru O, Spiridon AM, Belu I, Neamtu J, “Development and Validation of an HPLC Method for Simultaneous Quantification of Clopidogrel Bisulfate, Its Carboxylic Acid Metabolite, and Atorvastatin in Human Plasma: Application to a Pharmacokinetic Study” Hindawi Pub. Corp., 2015
38. Ravichandran S, Valliapan K, Ramanathan M, “Validated RP-HPLC Method for Concurrent Determination of Phenytoin Sodium and Clopidogrel Bisulphate in Tablet Dosage Form” J. Pharm. Sci. And res., 2015, 7(11), 934-937
39. Kumar VP, Sunandama Y, “Simultaneous Determination of Clopidogrel and Pioglitazone By High Performance Liquid Chromatography In Bulk Drug And Dosage Forms” Int. J. Pharm. And res. Sci., 2013, 2(1), 1-9
40. SWATHI SRI D, HEMANT KUMAR T, VARA PRASADA RAO K, SRINIVASA RAO Y, “Validated RP-HPLC method for simultaneous determination of rosuvastatin calcium and ezetembie in pharmaceutical dosage forum” International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491
41. Sandhya Donthula, Meriga Kiran Kumar, G. Shiva Teja, Y. Mohan Kumar, J.Yasodha Krishna and D. Ramesh, “A new validated RP-HPLC method for determination of Rosuvastatin calcium” ISSN 0975-5071.
42. Indian Pharmacopeia-2010, Indian Government Health and Welfare Society, Ghaziabad, pp 2072-2073
43. Kaila HO, Ambasana MA, Thakkar RS, Shah AK, “A New Improved RP-HPLC Method for Assay ofRosuvastatin Calcium in Tablets” Ind. J. Pharm. Sci., 2010, 72(5), 592-598
44. Pandya CB, Channabasavraj KB, Chadasam JD, “Development And Validation Of RP-HPLC Method For Determination OfRosuvastatin Calcium In Bulk And Pharmaceutical Dosage Form” Int. J. Pharm. Sci. Rev. And res., 2012, 5(1), 82-86
45. Donthula S, Kumar MK, Teja GS, Kumar YM, Krishna Y, “A new validated RP-HPLC method for determination of Rosuvastatin calciumin bulk and pharmaceutical dosage form” Der pharm. Let., 2011, 3(3), 350-356.
46. Rao AL, Suneetha D, “Development And Validation Of RP-HPLC Method For The Estimation Of Rosuvastatin In Bulk AndPharmaceutical Dosage Form” Int. J. Chem. sci., 2010, 8(2), 1308-1314
47. Kumar HT, Swathi SD, Rao PK, Rao SY, “Validated RP-HPLC Method For Determination Of Rosuvastatin Calcium In Bulk And Pharmaceutical Formulation” Int. j. pharm. Sci. and res., 2015, 6(7), 2913-2917.
48. Turabi ZM, Khatatbeh OA, “Stability-Indicating RP-HPLC Method Development and Validation for the Determination of Rosuvastatin (Calcium) In Pharmaceutical DosageForm” Int. J. Pharm. Sci. And drug res., 2014, 6(2), 154-159.
49. Swathi S, Kumar HT, Rao PK, “Validated RP-HPLC Method For Simultaneous Determination Of RosuvastatinCalcium And Ezetimibe In Pharmaceutical Dosage Form” Int. J. Pharm. And Pharm.sci. 2015, 7(4), 209-213.
50. Ramu K, Aleti P, Venisetty RK, “Analytical Method Development and Validation of Simultaneous Estimation of Ezetamibe and Rosuvastatin in Tablet Dosage FormBy RP-HPLC” Res. Art. Pharm. Sci., 2013, 3(4), 343-353.
51. Beludari MI, Prakash KV, Mohan GK, “RP-HPLC method for simultaneous estimation of Rosuvastatin and Ezetimibe from theircombination tablet dosage form” Int. J. Chem. And anal. Sci., 2013, 4, 205-209.
52. Varma D, Rao AL, Dinda SC, “Development And Validation Of Stability Indicating RP-HPLC Method For Simultaneous Estimation Of Rosuvastatin And Ezetimibe InCombined Tablet Dosage Form” Ras. J. Chem., 2012, 5(3), 269-279.
53. Kumar GV, Rajendra prasad Y, “Development and Validation of Reversed-Phase HPLC Method for Simultaneous Estimation ofRosuvastatin and Fenofibrate in Tablet Dosage Form” Int. J. Pharm. Tech. Res., 2010, 2(3), 2016-2021.
54. Thriveni J, Rambabu R, Rao JV, Vidhyadhara S, “Development And Validation Of RP-HPLC Method For Simultaneous Estimation of Rosuvastatin Calcium AndFenofibrate in Bulk And Pharmaceutical Dosage Forms” Int. J. Res. In pharm. And chem.., 2013, 3(2), 208-212.
55. Devika GS, Sudhakar M, Rao JV, “A New Improved RP-HPLC Method for Simultaneous Estimation of RosuvastatinCalcium and Fenofibrate in Tablets” Int. J. Pharm. And pharm. Sci., 2011, 3(4), 311-315.
56. Narayanker SM, Sakpal PH, Bhingare CL, Ingale PL, “Development and Validation of RP-HPLC Method for the Estimation ofRosuvastatin Calcium and Niacin in Combined Tablet Dosage Form” Int. J. Pharm. Res. And rev. 2015, 4(6), 44-50.
57. Tajane D, Raurale AM, Bharande PD, Mali AN, Gadkari AV, Bhoshle VR, “Development and validation of a RP-HPLC-PDA method for simultaneous determination of Rosuvastatin calcium andAmlodipine besylate in pharmaceutical dosage form” J. Chem. and pharm. Res., 2012, 4(5), 2789-2794.
58. Gousuddin MD, Sengupta P, Tripathi VD, Das A, “Stability Indicating RP-HPLC Method For Simultaneous Determination Of Aspirin And Clopidogrel In Dosage Form” Mal. J. Anal. Sci., 2016, 20(2), 247-257.
59. Shrivastava PK, Basniwal PK, Jain D, Srivastava SK, “Concurrent Estimation of Clopidogrel Bisulfate and Aspirin in Tablets by Validated RP-HPLC Method” Ind. J. Pharm. Sci., 2008, 70(5), 667-669.
60. Mahesh HR, Sudhakar KB, “A Novel Stability Indicating HPLC Assay Method for Simultaneous Estimation of Clopidogrel and Aspirin in Tablet dosage form by Using Core shell Technology column” Res. J. Pharm. And tech., 2015, 8(2)
61. Panda SS, “Ion-Pairing RP-HPLC Method for Simultaneous determination of Aspirin and Clopidogrel bisulphate in Tablet and Capsule Dosage Form” Int. J. Pharm. Tech. Res., 2010, 2(1), 269-273.
62. Londhe SV, Deshmukh RS, Malqund SV, Jain KS, “Development and Validation of a Reversed phase HPLC Method for Simultaneous Determination of Aspirin, Atorvastatin Calcium and Clopidogrel Bisulphate in Capsules” Ind. J. Pharm. Sci., 2011, 73(1), 23-29.
63. Devika GS, Sudhakar M, Venkateshwara RJ, “A New Simple RP-HPLC Method for Simultaneous Estimation of Aspirin, Atorvastatin and Clopidogrel in Capsule Dosage Form” Asian. J. Res. In chem.., 2011, 4(5), 795-799.
64. Gandala K, Lalitha R, Kishore K, Gopikrishna R, “A Validated RP-HPLC Method for Simultaneous Estimation of Aspirin and Rosuvastatin in Tablet Dosage Form” Int. J. Pharm. And chem. res., 2015, 1(3), 128-133.
65. Godavariya VD, Prajapati PB, Marolia BP, Shah SA, “Development and Validation Of RP-HPLC Method For The Simultaneous Estimation Of Rosuvastatin Calcium And Aspirin In Marketed Formulation” Int. Res. J. Pharm., 2012, 3(8), 173-175.
66. Solanki C, Patel N, “Development and Validation of RP-HPLC Method for Simultaneous Estimation of Rosuvastatin Calcium and Aspirin in Capsule Dosage Form” Int. J. Pharm. And bio. Sci., 2012, 3(3), 577-585.
67. Sheth A, Patel KN, Ramlingam B, Shah N, “Simultaneous Estimation Of Rosuvastatin Calcium And Clopidogrel Bisulphate From Bulk And Commercial Products Using A Validated Reverse Phase High Performance Liquid Chromatographic Technique” Int. Res. J. Pharm., 2012, 3(11), 154-157.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









