{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Patil N.B.*, Patil K.B., Wagh M.N., Patil A. A.
Department of Pharmacy,
Ahinsa Institute of pharmacy,
Dondaicha Tal-Shindakheda Dist-Dhule, Maharashtra, India
ABSTRACT
Indapamide is the Thiazide like diuretic drug used in treatment of Hypertension, as well as odema, Heart attack, stroke and heart failure in persons with high blood pressure. It is available in market single component and multicomponent formulations. The article summarizes 57 analytical method including the chromatographic method, LC-MS (Liquid Chromatography-mass spectroscopy), GC-MS (Gas chromatography-mass spectroscopy), spectriflurometric and UV Visible spectrophotometry techniques for estimation of Indapamide in biological samples, bulk and pharmaceutical formulation.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2633
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 12 Received On: 26/10/2018; Accepted On: 22/11/2018; Published On: 01/12/2018 How to cite this article: Patil, N.B., Patil, K.B., Wagh, M.N. and Patil, A.A. 2018. A review on analytical Method for Determination of Indapamide in Marketed pharmaceutical preparation. PharmaTutor. 6, 12 (Nov. 2018), 79-88. DOI:https://doi.org/10.29161/PT.v6.i12.2018.79 |
INTRODUCTION:
Indapamide is thiazide like diuretic drugs used in treatment of Hypertension, as well as odema, Heart attack, stroke, Heart Failure Patient with high blood pressure .Indapamide is an available in combination and single pharmaceutical dosage forms. [https://en.m.wikipedia.org, Tripathi K.D.2013]
Chemistry:-
Indapamide is diuretic class of drug .It is chemically 3-(amino sulfamoyl)-4-chloro-N-(2,3-dihydro-2-methyl-1H-indol-1-yl)benzamide.the molecular formula is C16H16ClN3O3S and molecular mass is 365.8 g/mol. Indapamide is white crystalline powder, odorless and it is soluble in methanol, ethanol, acetic acid, ethyl acetate and very slightly soluble in chloroform.[https://en.m.wikipedia.org]
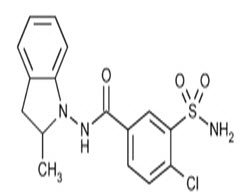
Fig.1. chemical structure of Indapamide
Pharmacology
1. Mechanism of Action:-
Indapamide Control Hypertension in part By inhibiting reabsorption of sodium (Na+) and chloride(Cl-) ions from distal convoluted in the kidneys by convoluted tubules in the Kidneys by blocking the Na+-Cl- symporter.[http:/www.sciencedirect.com]
2. Pharmacokinetics:-
Absorption of Indapamide from the gastrointestinal tract is rapid within 0.5 to 1 hour after an oral dose .It is plasma protein binding 79% and plasma elimination Half-life of Indapamide 16 Hours.[ http:/www.sciencedirect.com]
3. Dosage Forms & Recommended Doses:-
Oral Formulations of Inapamide Available in India .The recommended Dose single Daily 2.5mg. [ Tripathi K.D.2013]
4. Adverse Effect:-
In general most adverse effect are Dizziness,Headache,Fatigue,Muscle Cramps ,Asthenia ,GIT disturbances ,Electrolyte Imbalance (Hypokalemia ,Hypochloramia, Hyponatremia)[ https://en.m.wikipedia.org, Tripathi K.D.2013]
5. Contraindications:-
Indapamide is contraindicated in Sulphonamides, Severe Kidney Failure, Hepatic encephalopathy, low Blood Potassium level and pregnancy or Breastfeeding.
Analytical Method:-
This all are method which are used for determination of Indapamide in Pharmaceutical formulation and in biological fluids. This are all analytical method are reported during the literature survey. This all reported analytical method with specific condition. Indapamide is a commonly used in the treatment of Hypertension. the literature reports vast number of analytical methods such as UV spectrophotometry, HPLC( High performance liquid chromatography),HPTLC( High performance thin layer chromatography) ,GC-MS (Gas chromatography -mass spectroscopy),LC-MS (Liquid chromatography -mass spectroscopy) for the determination of Indapamide in Biological matrices, Bulk material and the pharmaceutical Dosage Formulation. The Objective this review to assemble the analytical method published for analysis of Indapmide in Biological matrices, Bulk and pharmaceutical formulation.
1. Spectrophotometry:-
In the literature survey were found that 12 UV spectrophotometric methods have been reported for estimation of Indapamide Single and in combined dosage form.
Table.1 illustrates the summery of the reported UV spectrophotometric methods indicating sample matrix used, Lambda Max, linearity range.
Table no.1 Summary of UV spectroscopic methods of Indapamide
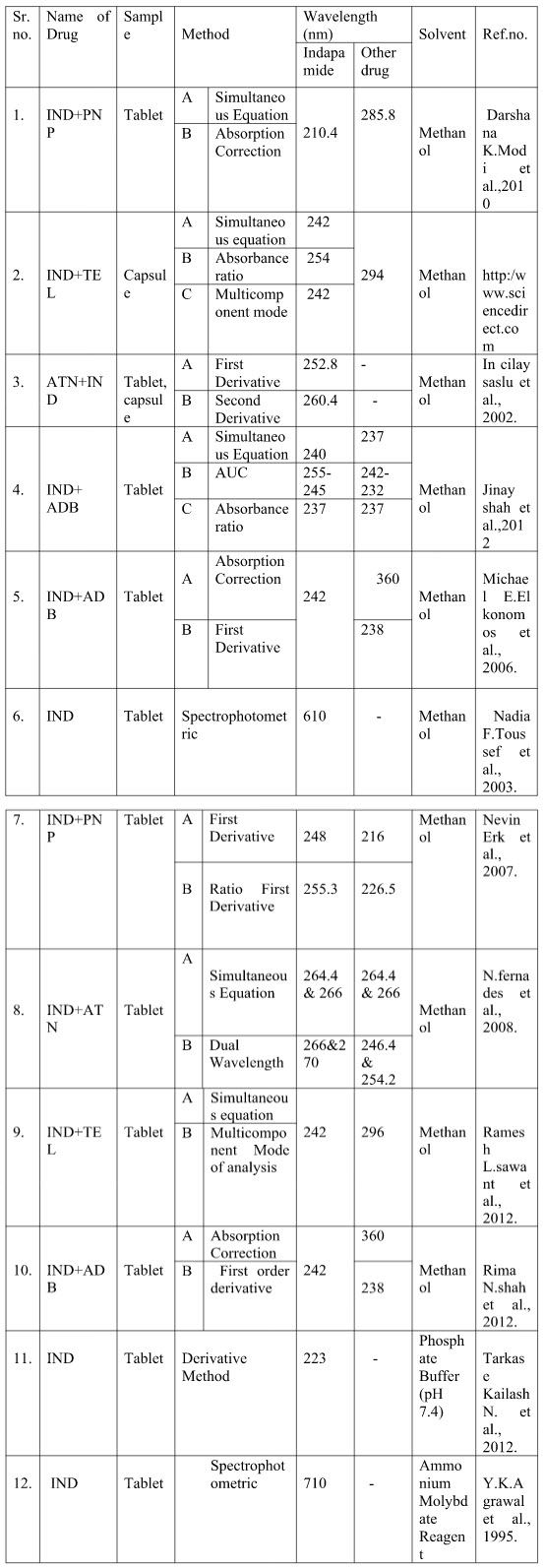
Where-
[AUC-Area under curve; ADB-Amlodipine Besylate; ATN-Atenolol; TEL-Telmisarthan; IND- Indapamide; PNP-Perindopril]
2. Chromatographic Method:-
The High performance liquid chromatography (HPLC) for residue determination of single and combination drug and also used in impurity profiling. High performance liquid chromatography (HPTLC) methods used in analysis of Indapamide in pharmaceutical dosage form.
Table no.2 shows the summarize reported chromatographic method indicating sample, method, mobile phase and wavelength.
Table no. 2 summary chromatographic method of Indapamide
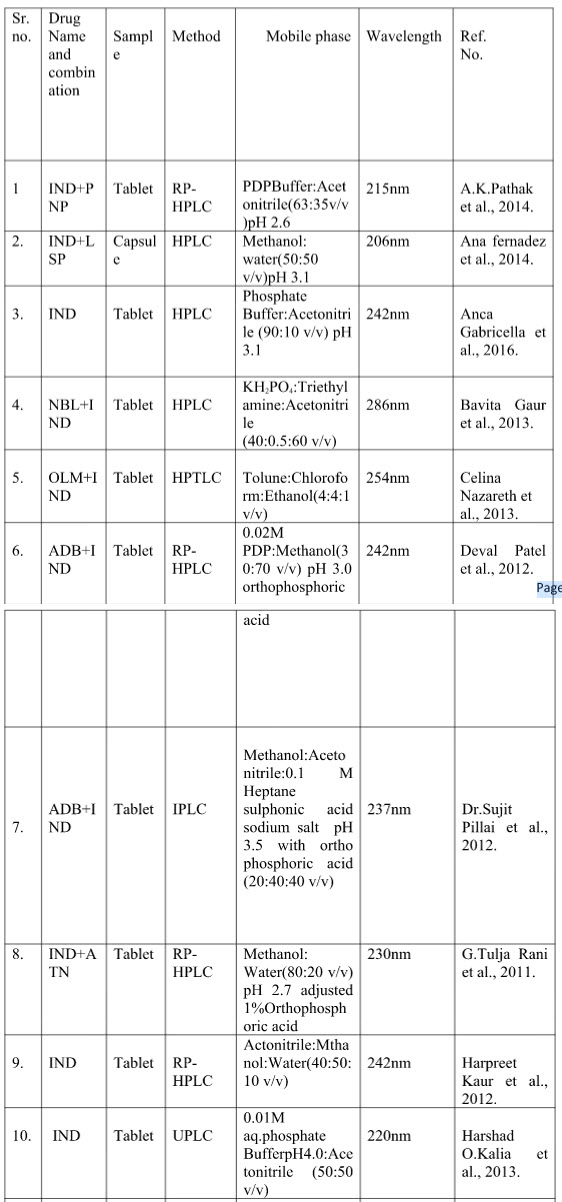
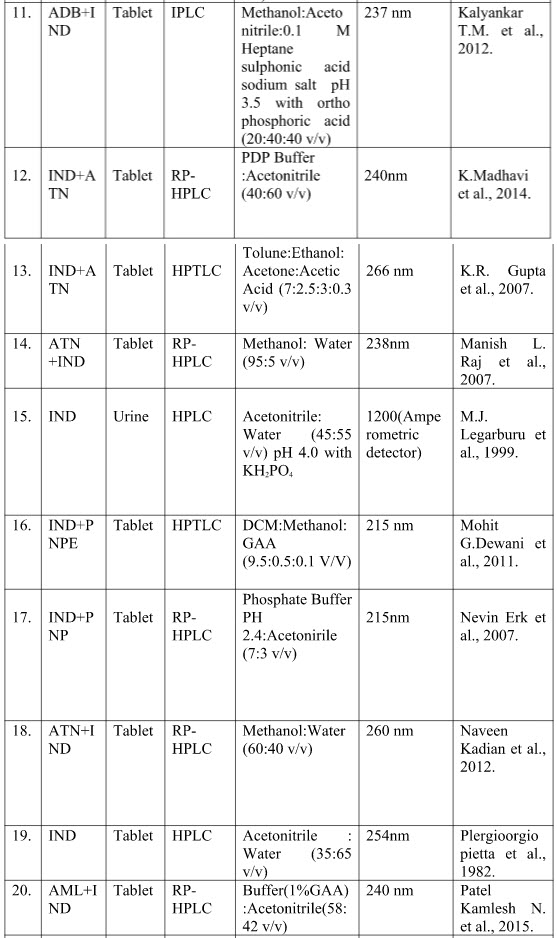
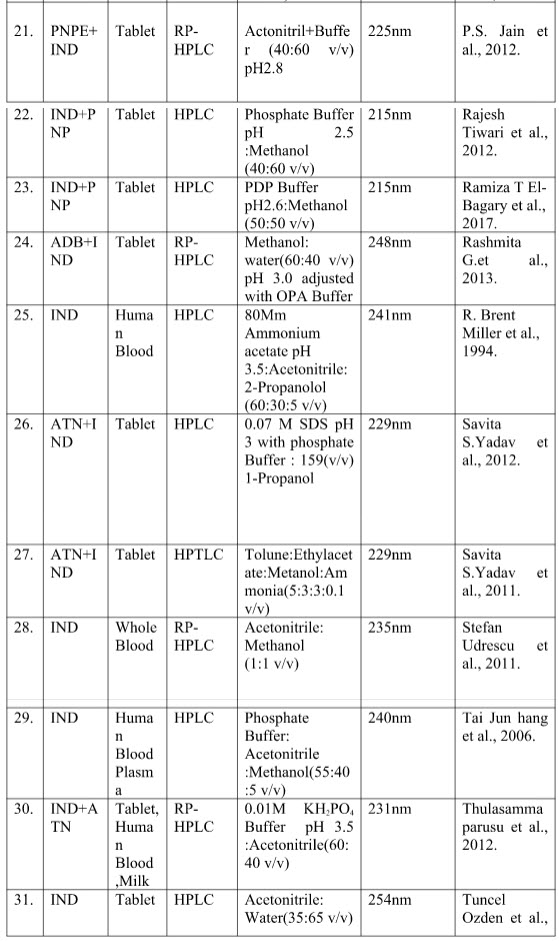
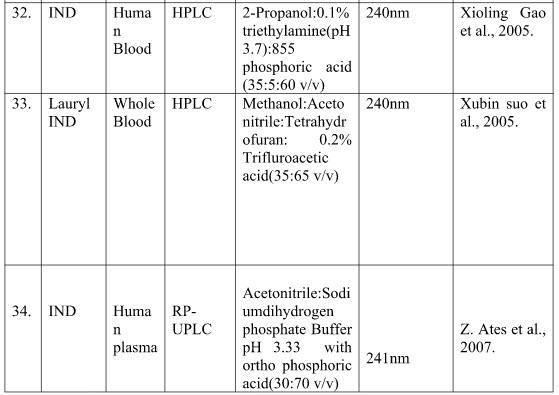
Where -
[IND-Indapamide; RP-HPLC- Reverse phase high performance liquid chromatography; HPLC- High performance liquid chromatography; HPTLC-High performance liquid chromatography; ADB-Amlodipine Besylate; AML-Amlodipine ;ATN-Atenolol; DCM-Dichloromethane ;GAA-Glacial acetic acid ;IPLC-Ion pair liquid chromatography ;KH2PO4-Potassium dihydrogen orthophosphate ;LSP-Lisinopril; NBL-Nebivolol OLM-Olmisarthan; OPA-Orthophosphoric acid; PDP-Potassium dihydrogen phosphate ;PNP-Perindopril ;PNPE-Perindopril Ebrumine ;RP-UPLC-Reverse phase –Ultra performance liquid chromatography ;SDS-Sodium dodecyl sulphate ;TEL-Telmisarthan ; UPLC-Ultra performance]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3. Spectroflurometric method:-
The spectroflurometric determination of Indapamide the first and second method are based on the oxidative coupling reaction of Indapamide with 3-methyl-2-benzothalidone hydrazine Hcl in presence of cerium ammonium sulphate in acidic medium and quenching effect on fluorescence of excess cerous ions at the emission max 350 nm and the excitation at max 300nm with linearity 1.2 to 9.6µg/ml with recovery 99.27% [Nadia F. Youssef et al., 2003.].
A sensitive fluorescence method for determination of Indapamide reaction of Indapamide with sodium hydroxide and addition of formaldehyde, linearity of 0.025 to 2.0 µg/ml the concentration of Indapamide of 0.05µg/ml can be detected in Dog given 20 mg of drug .the fluorescence at emission max 356nm and excitation max 284nm [Peter E.Grebow et al.,1978.].
4. GC-MS (Gas chromatography - mass spectroscopy) Method:-
A sensitive method of GC-MS of Indapamide SIM window 2.885 with separation Ions at 132,161,322,336,337,407 m/z & Dwell time is 40 [Claudio Brunelli et al.,2006.].
The determination of Indapamide in human urine using GC-MS & the retention time is 7.465 min. with segment range 7.2-7.9 min. and separation at Ions 407 m/z.[Olga Zaporozhets et al.,2012.].
5. LC-MS (Liquid chromatography-mass spectroscopy) Method:-
A sensitive method using LC-MS for the determination of indapamide in Human blood separation were performed on a symmetry c18 column (150×3.9mm id, 5µm) with mobile phase Acetonitrile: Water (60:40 v/v) Indapamide and IS(propyl paraben) were detected at m/z 364 for Indapamide and m/z 179 for propyl paraben with linear calibration range 2.0-120µg/ml.[Jingling Tang et al.,2005.]
A sensitive LC-MS method for the determination of Indapamide in human plasma using was separated on a C18 column with mobile phase 10mM Ammonium acetate: Methanol (22:78 v/v). Indapamide were estimated that by using Electrospray ionization the selected ions monitoring mode using target ions at m/z 364.3 for Indapamide and m/z 492.4 for the IS with linearity 0.1-100ng/ml for Indapamide. The mean recovery 90.5-93.95%. [Li Ding et al., 2006.]
A simple, sensitive and rapid LC-MS method for Quantification of Indapamide in human plasma the compound were separated on a stainless steel column (C18 shim-pack 5µm 150×2.0mm I.D., shimadzu) and separated ions monitoring at m/z 364.0 Indapamide and chlorpropamide m/z 275.0 with linear range 0.5 to 100ng/ml with a coefficient of determination(r) of 0.9998. [Yan Liang t al., 2006.]
6. Stability indicating method:-
Table no.3 summary stability indicating method of Indapamide
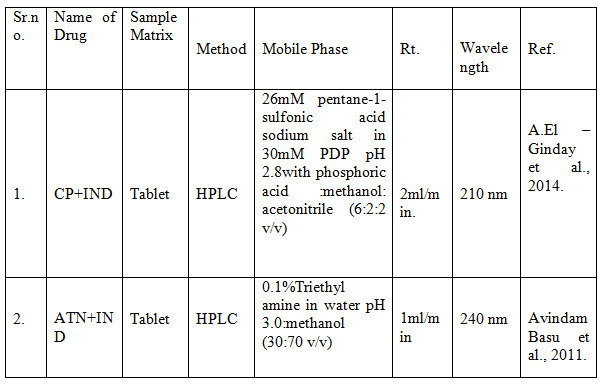
Where- [CP-Captopril; IND-Indapamide; ATN- Atenolol; HPLC- High performance liquid chromatography]
CONCLUSION:
This reviews articles presented the analytical methods for the estimation of Indapamide & its combination in pharmaceutical dosage form and biological sample like Blood, serum or plasma the literature survey of analytical data exhibit that HPLC methods are primarly for the analysis of Indapamide in single and in combination with other drugs in various formulation type of dosage form the other analytical methods like RP-HPLC, HPTLC, LC-MS, GC-MS, UV spectrometry,spectrofluorometry and stability indicating methods by HPLC used for the estimation of Indapamide in single and its combined dosage form, biological sample like blood,serum or plasma and milk. The presented information is useful for future prospective study for researcher in formulation development, Bio analytical research and Quality control of Indapamide.
REFERENCES:-
1. A.K. Pathak, Ruchana Lodhi , Mukesh K. Shikhri(2011);A Selective RP-HPLC method for determination of Perindopril and Indapamide in solid dosage form and bulk drug,JPR,4(8), 2512-2513.
2. Alaa El-Gindy, Mohammad Wafaa Nassar, Khalid Abdel Salem Attia, Hamed Abu-seada(2014); Stability indicating HPLC method for simultaneous determination of Captopril ,Indapamide and their related compounds; Journal liquid chromatography and related technologies; 37(5); 696-712.
3. Ana Fernandez Carballido, Emilia Bancia, Dumian cordoba Diaz, Sofia Negro (2014); Lisinopril-loaded Chitosan Nanoparticles and Indapamide in Hard Gelatin capsules: simultaneous HPLC Quantification ; Current pharmaceutical Analysis; 10(1); 10-19.
4. Anca Gabriela Carje, Valentin Ion, Alina Balinet, silvia Imre (2016); Enatioseparation of Indapamide by HPLC using Ovomucoid Glycoprotein as a chiral selector, farmacia,64(2), 181-186.
5. Avindam Basu, Bidyut Das, Krishnendu Basak, Mithun chakraborty(2011);Development and validation of stability indicating HPLC method for simultaneous estimation of Atenolol and Indapamide in tablet dosage form; IJR; 4(6); 1677-1680.
6. Bavita Gaur, Kalpesh Gaur, Karni Singh sekhawat and Anoop singh(2013);Development and validation of HPLC method for simultaneous estimation of Nebivolol and Indapamide in their combined tablet dosage form; World Journal of Chemistry; 18(1); 19-25.
7. Celina Nazareth, B. Shiva kumar, Prasad Reddy (2013); Development and validation of HPTLC method for simultaneous analysis of Olmesrthan, and Indapamide in bulk and combined dosage formulation, IMJPR; 3(12); 1709-1715.
8. Claudio Brunelli, Carlo Bicchi, Marco Vincenti (2006); High speed gas chromatography in doping control: fast GC and fast GC/MS determination of beta adrenoreceptor ligands and diuretics, J.Sep.Sci., 29,2765-2771.
9. Darshana k. Modi, chhagan N. Patel (2010); Development and validation of spectrophotometric method for simultaneous estimation of Perindopril and Indapamide in combined dosage form by absorbance correction method; IJPRIF; 2(1); 411-416.
10. Deval B. Patel, Falgun A. Mehta, Kashyap K. Bhatt (2012) ; Simultaneous estimation of Amlodipine Besylate and Indapamide by RP-HPLC method , Science pharm, 80(3); 581-590.
11. Dr.Sujit Pillai and Deepmala Manore (2012); Simultaneous spectrophotometric estimation of Telmisarthan and Indapamide in capsule dosage form; International J pharm; 4(1),163-166.
12. Eyad Rashed Dawud, Ashok K. Shakya(2014); HPLC-PDA Analysis of ACE-inhibitors Hydrochlorothiazide and Indapamide utilizing design of experiments; Arabian Journal of chemistry; 18,1-11.
13. G.Tulja Rani, D.Gowr i Sankar, P.Kadgapathi, B. Satyanaryana(2011);A validated RP-HPLC method for simultaneous estimation of Atenolol and Indapamide in pharmaceutical formulations, E-Journal of chemistry; 8(3); 1238-1245.
14. Harpreet kaur , H. Pannu, M.P. Mahajan, S. D. Sawant (2012);Validated RP-HPLC method for determination for determination of Indapamide in bulk and tablet dosage form, Derpharma chemica; 4(3); 996-1002.
15. Harshad O. Kaila, Mrunal A. Ambasana, Anamik K. Shah (2013);Development and validation of RP-HPLC method for determination of Six drugs used for combined Hypertension therapy, Journal of AOAC Int.; 96(2); 295-300.
16. https://en.m.wikipedia.org.
17. http./www.sciencedirect.com
18. Incilay Saslu, Scide Altinoz(2002); Two derivative spectrophotometric determination of Indapamide in pharmaceuticam dosage form; Journal of pharmaceutical and Biomedical Analysis; 30(2); 357-364.
19. Jinay shah, Parul Parmar, Mandev Patel (2012); Development and validation of spectrophotometric methods for estimation of Amlodipine Besylate and Indapamide in combined dosage form,JJPPS; 4(3); 257-261.
20. Jingling Tang, Jieli, Jin sun, Jing Yin, Zhonggus He(2005); Rapid and sensitive determination of Indapamide in Human blood by liquid chromatography with electrospray ionization mass spectrometric detection application to bioequivalence study,pharmazie; 60(11); 819-22
21. Kalyankar T.M., Khadkutkar P.K., Kakade R. B. (2012); Development and validation of Ion- pair liquid chromatographic method for the simultaneous estimation of Indapamide and Amlodipine Besylate in bulk and multicomponent formulation, IJRAP,3(5); 729-732.
22. K.Madhavi, K.Deepti, B. Havika (2014); RP-HPLC method development and validation for the simultaneous estimation of Atenolol and Indapamide in pharmaceutical tablet dosage forms; IJPAR; 3(1), 109-117.
23. K.R.Gupta, S.B.Wankhede, M.R.Tanjet and S.G.Wadodkar (2007); HPTLC estimation of Atenolol and Indapamide from Pharmaceutical dosage form ,Asian Journal of chemistry,19(6); 4183-4187.
24. Li Ding, Longhua Yang, Ningning Xiong(2006); A sensitive LC-ESI-MS method for determination of Indapamide in Human plasma ,method and clinical application, Journal of pharmaceutical and Biomedical analysis; 42(2); 213-217.
25. Manish L. Raj, Bharat G. Chaudhari (2012);Development and validation of RP-HPLC method for simultaneous estimation of Amlodipine Besylate and Indapamide in tablet dosage form; IJPSR,3(9); 3146-3150.
26. Michael E. El konomos, Ahmad A. Ahmad, Hesham Salem and Mahmoud A. Omar(2006); Spectrophotometric and spectrofluorimetric determination of certain diuretics in pure forms and their pharmaceutical formulation, Bull. Pharm. Sci; 29(1), 33-58.
27. M. J. Legarburu, R. M. Alonso, E. Ortiz(1999); Quantitative determination of Indapamide in pharmaceutical and urine by HPLC with Amperometric detection; Journal of chromatographic science; 37(8); 283-287.
28. Mohit G. Dewani, Kailash G. Bothara, Ashwini R. Madgudkar, Marinalini C. Damle (2011);Simultaneous estimation of Perindopril Ebrumine and Indapamide in bulk drug and tablet dosage form by HPTLC; pharmacie Globale (IJCP); 2(1); 1-4.
29. Nadia F. Youssef(2003);Spectrophotometric ,spectroflurometric and Densitometric methods for the determination of Indapamide, Journal of AOAC Int; 86(5); 935-940.
30. Nevin Erk (2007); Comparison of spectrophotometric and an LC method for the determination of Indapamide and perindopril in pharmaceuticals formulation, Journal of pharmaceutical and Biomedical Analysis, 26(1); 43-52.
31. N.Fernades, M.S.Nimdeo, V.P.Choudhari, R.R.Kulkarni, V.V. Pande and A.G. Nikalje(2008);Dual wavelength and simultaneous equation spectrophotometric methods for estimation of Atenolol and Indapamide in their combined dosage form, Int. J. chem sci; 6(1); 29-35.
31. Naveen Kadian, Meenaxi Marte, A. R. Bhat(2012);An effective RP-HPLC method for determination of Atenolol and Indapamide in Marketed tablet formulation; AJRC; 5(3); 405-408.
33. Olga Zaporozhets, Inna Tsyrutneva(2012); Determination of 8 Diuretics and probenecid in Human Urine by GC-MS spectrometry conformation procedure; AJAC, 3(4); 320-327.
34. Piergiorgio Pietta, Alma calatroni(1982); HPLC assay for monitoring Indapamide and its major metabolite in urine, Journal of chromatography, 228,377-381.
35. Patel Kamlesh N., Patel Nirav B.,Sevak Manan R.(2015); Development and Validation of RP-UPLC method of simultaneous estimation Amlodipine and Indapamide in their combined tablet dosage form, JPSBR; 5(1); 101-109.
36. Peter E. Grebow, Jo A.Treitman and Anne K.Yeung((1978); Fluor metric assay for Urinary Indapamide ,A Journal of pharmacy science; 67(8); 1117-1120.
37. P.S.Jain, P.R. Badreshiya, S.S.Chalikwar, A.A.Tondarwal, S.J.Surana(2012);Validation of dissolution method with RP-HPLC analysis for perindopril Ebrumine and Indapamide combination tablet,CI and CEQ; 18(1); 19-25.
38. Rajesh Tiwari, Anurekha Jain, Deepika Maliwal, E.Toppo(2012);Multicriteria optimization methodology in development of HPLC method for simultaneous estimation of Indapamide and Perindopril in bulk and combined dosage form, Asian J Pharm Clinical Res; 5(2); 50-53.
39. Ramesh L. Sawant, Manish A. Raskar, Raihan Ahmed and Sameer Pawar(2012);Validated spectrophotometric methods for simultaneous estimation of telmisarthan and Indapamide in pharmaceutical dosage form ,Derpharma chemica; 4(2); 633-638.
40. Ramiza I El -Bagary, Ehab f. Etkady, Maria A. Attallah (2017);A validated HPLC method for simultaneous determination of perindopril Arginine, Amlodipine and Indapamide :Application in bulk and in different pharmaceutical Dosage form, Journal of AOAC Into; 100(4); 992-999.
41. Rashmita G. ,Vasanth P.M., Ramesh T., Ramesh M.(2013);Simultaneous estimation and forced degradation studies of Amlodipine Besylate and Indapamide in tablet dosage forms by RP-HPLC method, Derpharma chemical; 5(6); 347-352.
42. R.Brent Miller, Durioush Dadgar and Marcel Lalande(1994); HPLC method for the determination of Indapamide in Human whole Blood; Journal of chromatography; 614; 293-298.
43. Rima N. Shah, Deesha B. Gandhi, Mehul M. Patel(2012); Simultaneous determination of Amlodipine Besylate and Indapamide in tablet dosage form by Absorption correction method and First order derivative UV spectroscopy; IJPTR; 4(3), 1018-1024.
44. Savita S.Yadav and Janhavi R. Rao(2012); Use of Miceller mobile phase for the chromatographic simultaneous determination of Atenolol and Indapamide in pharmaceutical Dosage form, International J pharm Bio science; 3(4); 645-655.
45. Savita S. Yadav and Janhavi R. Rao(2011); Simultaneous HPTLC analysis of Atenolol and Indapamide in tablet formulation, pharmacie Globale (IJCP), 2(09), 1-4.
46. Stefan Udrescu , Ialia Daniela Sora, Florin Albu, victor David(2011); Large volume of injection 1-Octanol as sample diluent in RP-HPLC: Application in bio analysis for assaying of Indapamide in whole Blood, Journal of pharmaceutical and Biomedical Analysis, ,54(5); 1163-1172.
47. Tai Jun Hang, wei zhao, Tie Liu, Ming Song, Ying Xie, Zheng Xing Zhang, Jiamping shem, Yingdi Zhang((2006); A selective HPLC method for determination of Indapamide in human whole blood: Application to bioequivalence study in Chinese volunteers, Journal of pharmaceutical and biomedical analysis; 40(1); 202-205.
48. Tarkase Kailash N., Jadhav Manisha B., Tajane Sachin R., Dongare Umesh S.(2012); Development and validation of UV-Spectrophotometric methods for estimation of Indapamide in bulk and tablet dosage form, Derpharma chemica,4(3),1128-1132.
49. Thulasamma Parusu and Venkateshwarlu Pooneri(2012);RP-HPLC method for simultaneous determination of Atenolol and Indapamide in pharmaceutical dosage form, human blood and milk, European Journal of chemistry, 3(2), 138-142.
50. Tripathi K.D.(2013);Essential of medical pharmacology, 7th edition , Jaypee Brothers medical publishers (P) Ltd.,Delhi.
51. Tuncel Ozden, Z. HazimTurker and A. Ulvi Tosun(1998); Quantitative HPLC analysis of Indapamide in pharmaceutical dosage form; Pharm pharmacol communication, 4; 397-399.
52. Xioling Gao, Jun Chen, Ni Mei, Xinguo Jiang(2005);HPLC determinations and pharmacokinetics study of Indapamide in Human Whole Blood, Chromatographia,61(11-12); 581-585.
53. Xubin suo,Yingjie Deng ,Aijun Hao(2005); Determination of Lauronyl-Indapamide in rat whole blood by HPLC; Journal of Chromatography B; 819(1); 191-196.
54. Yan Liang , Hao Li, wei Dong Chen(2006); Simple ,sensitive and Rapid LC-MS method for quantification of Indapamide in Human plasma application to pharmacokinetic studies, Analytical Letter; 39(7); 1365-1379.
55. Y.K. Agrawal, Mrs. F.D.Majumdar(1995);Spectrophotometric determination of Indapamide and its formulations using Ammonium Molybdate Reagent; Analytical Letters; 28(9;1619-1627.
56. Z. Ates, T.Ozden, S. Ozilhan,S.Eren(2007); Improved UPLC Determination of Indapamide in Human plasma; chromatographia; 66; 119-122.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









