{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
J. Y MANURE *, N. S NAIKWADE, P. J SHIROTE, S.A BAGWAN AND K. M KULKARNI
Department of Pharmaceutical Chemistry,
Appasaheb Birnale College of Pharmacy,
South Shivaji Nagar, Sangli- 416416, Maharashtra, India.
*javeedmanure.98600@yahoo.com
ABSTRACT
A new crystal form of 10-Propargyl-10-Deazaaminopterin, form SL, was discovered which is thermodynamically stable, shows long term stability and is applicable for using in final dosage forms. Form SL was characterized by several analytical methods such as thermal analysis by DSC, IR, X-ray powder diffraction, SEM (Scanning Electron Microscopy), Digital optical microscopy and solubility/dissolution measurements. Active pharmaceutical ingredients should be stable during technological processes for the preparation of a final dosage form and must also remain stable during storage. The morphology of long needle and plate shaped crystals was investigated by scanning electron microscopy (SEM) and optical microscopy. Form SL was compared to other forms of 10-Propargyl-10-Deazaaminopterin such as form A, B, C and to the amorphous form. The thermodynamic stability of form SL is supported by its long-term stability. Form SL is a pharmaceutically applicable crystal form.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2573
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 4 Received On: 08/01/2018; Accepted On: 27/01/2018; Published On: 01/04/2018 How to cite this article: Manure JY, Naikwade NS, Shirote PJ, Bagwan SA, Kulkarni KM; A New Types of Polymorphic Form of 10-Propargyl-10-Deazaaminopterin, from SL: As a Refractory Peripheral T-cell Lymphoma Drug; PharmaTutor; 2018; 6(4); 45-50; http://dx.doi.org/10.29161/PT.v6.i4.2018.45 |
INTRODUCTION
Pralatrexate or 10-Propargyl-10-Deazaaminopterin is the generic name of the compound (2S)-2-[[4-[(1RS)-1-[(2,4-diaminopteridin-6-yl)methyl]but-3ynyl]benzoyl]amino] pentandioic acid Fig.1 which belong to the field of pharmaceutical agents for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. 10-Propargyl-10-Deazaaminopterin (Pralatrexate) is an antifolate and acts as an inhibitor of dihydrofolatereductase.
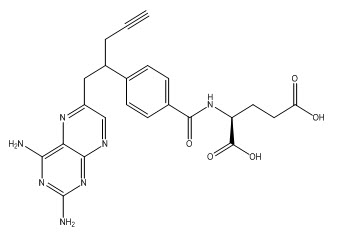
Figure 1. Chemical Structure of Pralatrexate
A New polymorphic form of a pharmaceutically useful compound also provides an opportunity to improve the performance characteristics of a pharmaceutical product. Discovering different polymorphic forms of a pharmaceutical product can provide materials having desirable processing properties, such as ease of handling, processing and purification, storage stability. It enlarges the choice of materials that a formulation scientist has available for formulation optimization, for example by providing a product with different characteristics, e.g. better processing or handling characteristics, improved dissolution profile, or improved shelf life. The synthesis of the compound was first described in the 1993’s. (DeGraw et al., 1993) Sirotnak et al. reported disclosed 10-Propargyl-10-deazaaminopterin substantially free of 10- deazaaminopterin. (Sirotnak et al., 2000) Gijsbertus J. Pronk reported pure diastereomers of 10-propargyl-10-deazaminopterin, or a salt thereof, wherein the diastereomer may be either (2S)-2-[[4-[(1S)-1-[(2,4-diaminopteridin-6-yl)methyl]but-3-ynyl]benzoyl]amino]pentanedioic acid or (2S)-2-[[4-[(1R)-1-[(2,4-diaminopteridin-6-yl)methyl]but-3-ynyl]benzoyl]amino]pentanedioic acid.
(Gijsbertus J, 2011). The Polymorphs with different crystalline characteristics have become the subject of extensive solid-state studies over the past few years, primarily due to their potential impact on the development of active pharmaceutical ingredients and the final dosage form. (Carstensen, 2001). An active pharmaceutical ingredient in the final dosage form and to control the manufacturing process, both polymorphism and pseudo-polymorphism should be investigated during the pre-formulation phase of the development in order to assure the right bioavailability and stability of API. (Morris, 1999). Occurrence of different crystal forms of same substance i.e. polymorphism, is a property of some molecules and molecular complexes. A single molecule for e.g. 10-Propargyl-10-Deazaaminopterin, may give rise to a variety of polymorphs having distinct crystal structures and physical properties like XRPD pattern, melting point, TGA, DSC and infrared absorption spectrum. Different polymorphic forms of a compound can be distinguished by using one or more of these techniques.
I discovered a new crystalline polymorphic form of Pralatrexate, form SL, which is thermodynamically stable, shows long term stability and is applicable for using in final dosage forms. Form SL was characterized by several analytical methods such as thermal analysis by DSC, IR, X-ray powder diffraction, SEM (Scanning Electron Microscopy), Digital optical microscopy and solubility/dissolution measurements. Active pharmaceutical ingredients should be stable during technological processes for the preparation of a final dosage form and must also remain stable during storage.
For convenience, a perspective drawing together with the different atomic molecular structure is depicted in Fig. 2
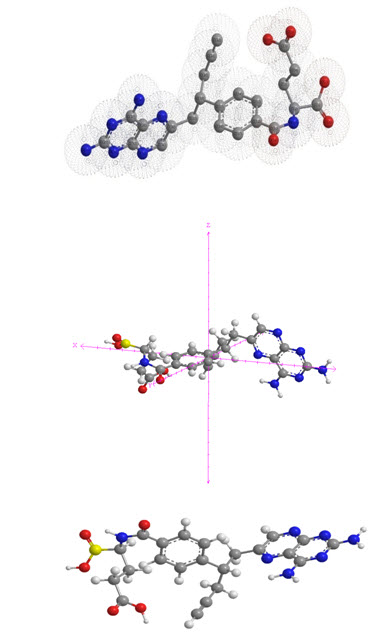
Figure 2. The molecular structure of Pralatrexate in X, Y and Z axis plane.
MATERIAL AND METHODS
Pralatrexate as a starting material for crystallization studies is a semi-solid, pitchy, oily or amorphous material. It was prepared by chemical synthesis and purified using chromatographic methods. Form A, B and C was novel procedure described in WO 2012/061469. (Tiseni, 2013) According to these procedure amorphous Form A, B and C was prepared by dissolving pralatrexatein formamide at room temperature. The solution was poured into water at room temperature resulting in the formation of a precipitate. The precipitate was filtered off and dried in a vacuum drying oven at 35°C under vacuum for 18 h, furnishing pralatrexate Form A (200 mg, 50% yield, 96% purity).
From SL prepared by 6 to 7 mL DMSO was placed in to a 50 ml RBF at 25-30°C. Further at same temperature, 100 mg 10-Propargyl-10-Deazaaminopterin was added to the reaction mixture. Reaction mixture is stirred for 10-15 minutes. After obtaining the clear solution, the reaction mass was heated to 50-55 °C and stirring was performed for 30 min. This was followed by slow addition of 10 mL acetone. Reaction mass was further stirred for 30 to 50 min. Then slowly cooled the reaction temperature to 25-30°C, where stirring was performed for 16 to 20 h. After completion of the reaction, the reaction mass was filtered through Buckner funnel. The wet cake obtained was further washed with 2.0 mL acetone. The material obtained was suck dried, unloaded and dried for 30 mins at 25-30°C by air drying to afford 60 mg crude 10-Propargyl-10-Deazaaminopterin (I) crystalline polymorphic Form- SL. The Purification of crude 10-Propargyl-10-Deazaaminopterin crystalline polymorphic Form- SL is prepared as 50 mg of above obtained material is charged in to a 20 ml RBF at 25-30°C. Further 3 to 6mL acetone was added to the RBF. Reaction mass was stirred for 10-15 minutes, followed by heating to 50-55 °C with maintain the temperature for 30 mins along with continuous stirring. Then reaction mixture was slowly cooled to the temperature of 25-30 °C, where stirring was performed for 1 to 3 h. On completion of stirring the reaction mass was filtered through Buckner funnel and the wet cake obtained was washed with 1.0 mL acetone. The wet material is unloaded and suck dried for 10-15 min. This reaction sequence was repeated twice to achieve the desired purity level. Final material obtained is dried under vacuum at temperature of 60-65°C for 16h to afford 10 to 15 mg of pure 10-Propargyl-10-Deazaaminopterin (I) crystalline polymorphic Form- SL which is characterized by M.P. of 221-224 °C.
Thermal analysis:
Thermal Analysis DSC was performed on a Mettler DSC821e. The sample (4–6 mg) was placed in an unsealed aluminum crucible with a hole and heated in an N2 atmosphere at a heating rate of 5 K/min in the temperature range from 30 °C to 200 °C. Indium was used to calibrate the instrument.
FT-IR Spectroscopy:
FT-IR spectroscopy was obtained on FT-IRspectrometer and the infrared spectra were recorded within the wave number range of 4000–400 cm–1 with a Perkin Elmer FT-IR spectrometer at a resolution of 2 cm–1. Dry KBr (50mg) was finely ground in an agate mortar and sample of the drug (1-2 mg) was subsequently added and mixed gently.
X-Ray powder diffraction:
The powder x-ray diffraction patterns were obtained using a Panalytical X’Pert PRO diffractometer with an X’Celerator detector (RTMS; Real Time Multiple Strip) using CuKα radiation (tube operating at 45 kV and 40 m-A) in the Bragg-Brentano (reflection) geometry. Data were recorded from 2 to 40° 2θ in steps of 0.033° 2θ with a measurement time of 50 seconds per step. Variable divergence and antiscatter slits were used to maintain 10 mm of sample length irradiated. Major diffraction peaks were extracted from the diffraction patterns using X’Pert High-Score Plus 2.0 software.
Digital Optical Microscope:
The optical images of crystals were obtained using a Triangular microscope and digital micro-imaging adaptor (SAGLO Research Equipments SGL-11 Digital Micro-Imaging Adaptor as a Digital microscope Camera and SAGLO soft Image Analysis Software, Indian Design Patent No. 263618) operating at magnification was 200X.
Scanning Electron Microscopy:
A Jeol JSM-6100 scanning electron microscope was used to obtain photomicrographs of 10-Propargyl-10-Deazaaminopterin and its crystal modifications. Electron images of crystals were obtained using a scanning electron microscope (Jeol JXA-840A) operating at 18 kV. The magnification was 500X. The specimens were mounted on a metal stub (with double-sided adhesive tape) and coated with gold under vacuum prior to observation.
RESULTS AND DISCUSSION
Polymorphic Screening:
Five crystalline forms were screened from saturated solutions of 10-Propargyl-10-Deazaaminopterin (Form SL) which were prepared by heating in different solvents and solvent mixtures under constant stirring until most of drug has dissolved; the solutions were filtered and kept at room temperature until crystallization was complete.
Dsc Thermograms:
Fig. 3 shows DSC thermograms of the crystal forms and amorphous form of Pralatrexate. There are no endothermic peaks at temperatures below the melting points. It can be easily concluded from the DSC analysis that the crystal forms of Pralatrexate are unsolvated forms. The melting points and the melting enthalpies were obtained from the DSC thermograms and are presented in Table 1.
Table. 1 Melting points and enthalpies of the crystal forms of Pralatrexate
|
Crystalline form |
Onset melting point 0C |
Peak 0C |
Melting enthalpy (J/g) |
|
Form SL |
230.0 |
126.8 |
-69.8 |
|
Amorphous Form |
120.0 |
110.2 |
-56.8 |
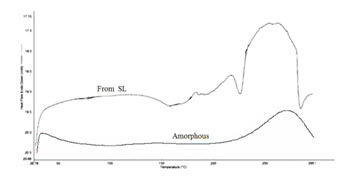
Figure. 3 DSC thermograms of amorphous and SL forms of Pralatrexate.
Due to differences in sample purity, particle size distribution, and the presence of residual solvents cause some variations and broadening of the melting range. Form SL has the highest melting point and amorphous form the lowest. Form SL is thermodynamically the second most stable form of Pralatrexate. A wide endothermic peak at the temperature above the melting point shows the decomposition of Pralatrexate.
FT-IR Spectroscopy:
FTIR has been successfully used for exploring the differences in molecular conformations, crystal packing and hydrogen bonding arrangements for different solid-state forms of organic compounds. 10-Propargyl-10-Deazaaminopterin (I) crystalline Form-SL shows IR absorption spectrum and it is characterized by peaks expressed in cm-1 at approximately 3294 cm-1, 3205 cm-1, 2114 cm-1, 1645 cm-1, 1555 cm-1, 1538 cm-1 and 1499 cm-1 is shown in Fig. 4
Figure 4. FTIR of Form A, Form B, Form C and Form SL of Pralatrexate
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
X-RAY Powder diffraction:
The powder x-ray diffraction for 10-Propargyl-10-Deazaaminopterin crystalline form-SPR, characterized by X-ray powder diffraction pattern consists of characteristic 2θ° peaks selected from the XRPD peak set of 8.22, 11.93, 15.84, 16.23, 16.33, 17.44, 17.66, 18.34, 18.55, 18.89, 19.02, 24.45, 25.27 and 25.53 ± 0.2 2θ° is shown in Fig. 5.
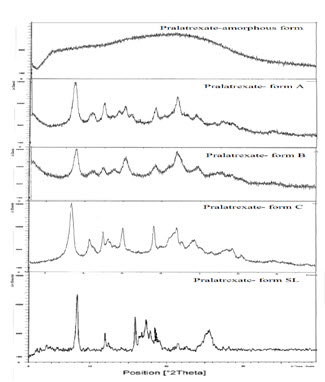
Figure 5. X-ray powder diffraction pattern of 10-Propargyl-10-Deazaaminopterin crystalline Form-SL.
The characteristic peaks and their d spacing values of the new crystalline Form-SL are tabulated in the Table-2.
Table. 2 The characteristic peaks and their d spacing values of the new crystalline Form-SL.
|
Angle (2θ°)±0.20 |
D Spacing Value (A°) |
|
8.22 |
10.679 |
|
11.93 |
7.408 |
|
15.84 |
5.567 |
|
16.23 |
5.476 |
|
16.33 |
5.354 |
|
17.44 |
5.144 |
|
17.66 |
4.934 |
|
18.34 |
4.867 |
|
18.55 |
4.755 |
|
18.89 |
4.711 |
|
19.02 |
4.633 |
|
24.45 |
3.655 |
|
25.27 |
3.599 |
|
25.53 |
3.482 |
Digital Optical Microscope:
The optical microscopic image of crystals Form SL was found to exist in long plate shaped crystals is shown in Fig. 6.
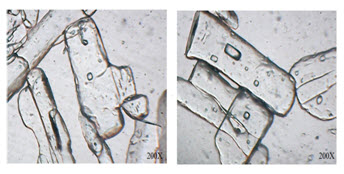
Figure 6. Optical microscopy of Form SL of 10-Propargyl-10-Deazaaminopterin
Scanning Electron Microscopy:
The difference in morphology was confirmed by studying the shape and appearance of different polymorphic forms of 10-Propargyl-10-Deazaaminopterin crystalline Form-SL by scanning electron microscopy is shown in Fig. 7.
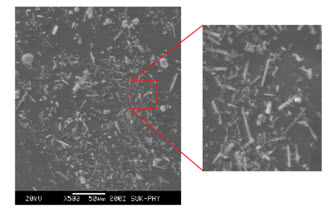
Figure 7. Scanning electron microscopy of Form SL of 10-Propargyl-10-Deazaaminopterin.
Form SL was found to exist in long needle and plate shaped crystals while the photomicrograph. The clear cut differences in the photomicrographs of above forms suggest them to be morphologically different forms. Further analysis by other analytical techniques was performed to confirm these forms to be different forms.
CONCLUSION
In conclusion, a novel crystal form of 10-Propargyl-10-Deazaaminopterin, form SL, was discovered during the study of polymorphism of various crystal forms of 10-Propargyl-10-Deazaaminopterin and its preparation from halogenated aromatic solvents (solvates). The new crystal form was characterized by various analytical methods (XRD, FTIR, DSC, SEM, and Optical microscopy). Form SL is thermodynamically stable. Its long-term stability testing using several stability programs showed no solid-state transitions. Due to the crystal form, shape, size, and particle size distribution, it is easily transferable into the drug manufacturing process. It to be least crystalline in nature and can be further utilized for formulation development.
REFERENCES
1. DeGraw et al. J. Med. Chem, 1993, 36, 2228.
2. Francis M. Sirotnak, James R. Piper, Joseph I. DeGraw, William T. Colwell. US Patent 6028071B2, 2000 22 Feb.
3. Gijsbertus J. US patent application 2011/0190305A1, 2011 Aug 4.
4. J. T. Carstensen, Marcel Dekker, New York, 2001.
5. K. R. Morris, In:Polymorphism in Pharmaceutical Solids. Marcel Dekker, New York, 1999.
6. Paolo Simone Tiseni and Tomislav Biljan, WO 2012/061469, 2013 May 2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









