

About Authors
Praveen Kumar. K*1, Sudhir KK1, Akshay KA1, Sachin KK1, Shripathy. D2, Shabaraya AR3.
1M Pharm, Department of Pharmaceutics, Srinivas College of pharmacy, Mangalore- 574143)
2Associate professor, Department of Pharmaceutics, Srinivas college of Pharmacy)
3Principal and HOD of Pharmaceutics, Srinivas college of pharmacy, Mangalore)
* kodavanjipraveen@gmail.com
ABSTRACT
Pharmaceutical nanosuspensions is an emerging techniques for insoluble drugs to make it as soluble. More than 40% of the new drugs are being produced through drug discovery programs are poorly water-soluble compound. Formulating a poorly water soluble drug has always been a challenging problem to pharma industry. The techniques like bottom up and top down methods. Solvent, stabilizers and preservatives are very essential for the formulation of nanosuspensions. The particle size in the range of 100-600nm, Percentage drug encapsulation efficiency (%EE) and zeta potential in the range of -25 to +25mV. The other evaluation parameters like X-ray diffraction study, kinetic release, were also performed based on the type of formulations.
INTRODUCTION
Generally the active pharmaceutical ingredient are very important in the preparation of pharmaceutical formulations. But more than 40% of the active pharmaceutical ingredients are synthesised through drug discovery programmes that are water insoluble or lipophilic drugs. The formulation of a poorly aqueous soluble form can be converted into soluble form is a challenging problem by the pharmaceutical scientist. The innovated formulation contained nano-sized particles can be manufactured to all drug compounds belonging to BCS classes II and IV. To enhance their solubility and hence penetration of drug from gut to the systemic circulation by the gastrointestinal barrier.1
Basically, the rate-limiting step for absorption of the drugs in the above classes is the dissolution velocity arising from low solubility. Although the drugs are high permeability, the poor solubility results in a low concentration gradient between gut and blood vessel consequent to a problem of drug transport and oral absorption. Now a days, there are a large percentage of drug compounds in drug development represents as poor aqueous solubility. Therefore, one of the most challenging tasks in drug development is to improve the drug solubility in order to enhance the bioavailability of these drugs.2
There are many existing methods for enhancing the solubility of poorly aqueous drugs, which include micronization, co-solvency, salt form, using surfactants, precipitation technique. Other techniques such as liposomes, emulsions, micro-emulsion, solid dispersion, and inclusion cyclodextrin complex can enhance the drug solubility markedly, but they could not possible to all drugs. However, these methods have their own drawbacks such as a large amount of additives that may induce stability and toxicity issues. Therefore, they are frequently not ideal for clinical treatment.3since, nanosuspension drug delivery system was firstly developed in 1994, and nanosuspension has attracted more attention as a formulation for the poorly aqueous soluble drugs.4
Pharmaceutically developed nanosuspension can be defined as a very finely colloidal or biphasic, dispersed or solid drug particles in aqueous vehicle dosage form, particle size of range less than 1µm, without any matrix material, stabilised by using suitable surface active agents &stabilizers, prepared by suitable methods for the drug delivery applications based on the site of application like oral, topical, parenteral, ocular & pulmonary routes. This type of formulations not only solves the problem of poor aqueous solubility & drug availability in the body and also alters the pharmacokinetics of drug & that improves safety & efficacy. This advanced approach is most suitable for the compounds with more log P value, great melting point & more initial dose. More drug availability in the body when comparing to the existing methods of drug delivery. The decreased in particle size leads to an enhanced in the surface area & absolutely increased in the rate of dissolution as described by Nernst-Brunner & Levich modification or Noyes-Whitney equation. In addition, slightly increased in saturation solubility is depended by particle size reduction due to an increase dissolution pressure. This can be explained by the Ostwald-Freundlich equation. Depending on the production technique applied changes in crystalline structure of the drug particles may also occur. An increasing amount of amorphous drug fraction could produce higher saturation solubility. Furthermore, a general adhesiveness to the tissue has been described for nano-sized particles.5
These types of innovations, the drug is to be maintained as a crystalline state with reduced particle size, leading to increase dissolution rate & therefore improved bioavailability. The encapsulated form of drug within nanosuspension can exist in pharmaceutically accepted crystalline or amorphous state. In this novel technique can successfully formulate the brick dust molecules for improved dissolution & good absorption.6
Criteria for selection of host molecule as a nanosuspension : 7
In this method the API that is having either of the following characteristics like,
a) Non aqueous drug molecule having good solubility in the oils.
b) Non aqueous active pharma ingredient have insoluble in both water and oils.
c) Drug molecules having the tendency of the lesser crystals to dissolve the solvent.
d) Large initial dose molecules.
Advantages : 8
1. Nanosuspensions were more stable than lipoidal carriers.
2. Provide ease of manufacture and scale up for large scale production.
3. Tissue inconvenience can be reduced by formulating as a nanosuspension.
4. Enhanced bioavailability in ocular and inhalation route.
5. Parenteral (IV) route can be achieved rapid onset of action. & tissue targeting.
6. Solid dosage form containing nanosuspension to produce improved bioavailability.
7. Duration of action can be increased, due to submicronic level of particle size.
8. Drug with higher penetrability value can be formulated as nanosuspension to increase the bioavailability as well as the duration of action.
9. High dissolution rate can be achieved due to improved tissue performance & saturation solubility of the drug molecules.
10. It can be converted in to suitable dosage forms like, tablets, capsules, pellets, hydrogel & suppositories based on the requirement of formulator.
11. The amorphous fraction of the particles increases leading to a surface charge in the crystalline structure leads to higher solubility.
12. Surface-modification of nanosuspension is one of the possibility to target site specific.
Disadvantages for Nanosuspension Drug delivery system :
1. Physical instability is one of the main drawback in this type of formulation.
2. The care must be taken during handling & transport, because the preparation could be bulk.
3. Dose fixation could be difficult.
Methods for the preparation of nanosuspension: 9, 11, 16, 24, 26, 27
The techniques for the preparation of nanosuspension can be broadly classified in to four methods like,
I. Bottom up techniques
II. Top down techniques
III. Combined techniques
IV. Other techniques

Fig no: 01 Methods for nanosuspension preparation
I Bottom-up technology:
It is the oldest method. In this method, drug or host molecule can be dissolved in the organic solvent and could be injected through the aqueous solvent. The entire solution can be stabilized by suitable stabilizers.
Advantage :
1) Use of simplest and common methods.
2) Higher saturation solubility is one of the advantage for precipitation compared to other method of preparation.
Disadvantages :
1) Drug solubility is the main criteria.
2) The solvent miscibility with at least one non-solvent is to be required.
3) Complete removal of solvent residues is another one of the challenging step, thus increasing production costs.
4) The preservation of particle character is little bit difficult (i.e. size, in case of amorphous fraction). It can be done by using spray dryer as a second step process for the particle size preservation.
Eg: Precipitation ultrasonication method

Fig no : 02 Precipitation ultrasonication methd
II Top-Down Technology:
The top down technologies include,
a) Media milling method :

Fig no: 03 Media milling method
b) High pressure homogenization method :
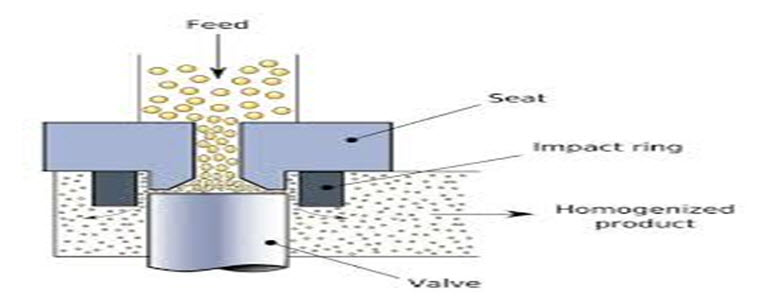
Fig no : 04 High Pressure Homogenization (HPH)
III Combination methods:
1.0 Emulsion Diffusion Method :

Fig no: 05 Emulsion diffusion method
2.0 Micro-emulsion Template;

Fig no: 06 Micro emulsion template method
IV Newer methods:
i. Supercritical Fluid Method;
Supercritical fluid technology is the advanced method to prepare nanoparticles from drug solutions. The Rapid Expansion of Supercritical Solution Process (RESS). It involves the passage of supercritical fluid containing drugs through a nozzle, which leads to the precipitation of the nano sized fine particles by evaporation of solvents.

Fig no: 07 Super critical fluid method
ii Dry Co-Grinding;
It is the modified techniques of conventional milling method. This method is applicable in case of poorly soluble drugs with soluble polymers and Copolymers can be dispersing in a liquid or a suitable media. Polymers like PVP, Polyethylene glycol (PEG), Hydroxypropyl methylcellulose (HPMC) and Cyclodextrin derivatives have been used in the preparation of nanosuspensions.
Components of nanosuspension formulation: Nanosuspension formulation requires basically stabilizer or surfactant, proper solvent system and others ingredients for its preparation7.
Table no : 01 Ingredients required for nanosuspension formulation
|
SL NO:
|
Ingredients
|
Role of the ingredients in the formulation
|
Examples
|
|
01
|
Stabilizers
|
It can be used to reduce the surface free energy or aggregation or Surface tension that can be essential for the physical stability of pharmaceutical nanosuspension.
|
Polysorbates, Povidone, Poloxamer and lecithin
|
|
02
|
Solvents
|
Organic solvent are more common, but they are less hazardous in nature.
Must solubilize the active pharmaceutical ingredient.
|
Methanol, Ethanol, DMSO, Chloroform.
|
|
03
|
Preservatives
|
The entire formulation can be protected from the microbial attack.
|
Benzoic acid, Methyl or Propyl parabens.
|
|
04
|
Co-surfactants
|
They are influence phase behaviour. Therefore the effect of co-surfactant on uptake of the internal phase or drug loading should be investigated
|
Transcutol, Bile salts, Isopropanol.
|
|
05
|
Other additives
|
Uses of other ingredients mainly depends upon either the route of administration or physicochemical properties of drug.
|
Buffers, Salts, Polyols, Osmogenes and Cryoprotectant
|
Characterisation of nanosuspension formulations:
Nanosuspensions are characterized for physical appearance, Drug excipient interactions studied by FTIR, particle size, zeta potential, crystalline status, dissolution studies and in vivo studies. Among this, the most important characterization techniques were discussed.
Pre formulation studies:
• Physio chemical properties of drug and excipients can be evaluated. Pre formulation studies like Colour, Melting point determination, Solubility studies in different solvents, FTIR study.
• Melting point determination:
Melting point can determined by capillary method. The sample was inserted in capillary tube having one end closed. Then the capillary was inserted in Thiele’s tube which was heated in controlled manner. The temperature at which drug sample started melting was noted as melting point temperature.
• Solubility studies:
Solubility is based on the type of drug used in the preparation. Nanosuspensions have an important advantage over other techniques, that it can increase the dissolution velocity as well as the saturation solubility. The saturation solubility of the drug in different physiological buffers as well as at different temperatures should be assessed using methods described in the literature.
Post formulation study:
1. Viscosity determination:
It can be studied by using Brookfield rotary viscometer at room temperature
2. Particle size analysis and zeta potential:
It can be studied by using Malvern zeta sizer. This parameter is very important. The average particle size range is 200-600nm
3. Percentage drug entrapment efficiency:
Methanol is used as a standard solvent used to break the nanoparticles, after centrifugation to get the entrapped and free drug. Remove the unentrapped/free drug. The % EE is calculated by using the formula,
% Entrapment efficiency=Drug added - Free drug / Total drug *100
4. X-ray diffraction study:
It is used to study the nature of the crystals of nanoparticles
5. In vitro drug release:
Nanosuspension is placed on one side of the cellophane membrane in a vertical Franz diffusion cell. Other side of the membrane was in contact with the dissolution medium. Entire dissolution assembly was placed on a magnetic stirrer at temperature of 37°C. The dissolution medium was withdrawn at different time intervals- 1hr, 2hr, 3hr, 4hr, 5hr, 6hr ,. Whenever sample was withdrawn equal volume of fresh dissolution medium was added to the cell to maintain a constant volume. Drug concentrations in the dissolution medium were determined by UV spectrophotometric method
6. Stability of Nanosuspension:
The high surface energy of nanosized particles induces agglomeration of the drug crystals. The main function of the stabilizer is to wet the drug particles thoroughly to prevent Ostwald ripening and agglomeration of the Nanosuspension and form a physically stable formulation by providing a steric or an ionic barrier.
The evaluation parameters can be changed based on the type of formulations.
Therapeutic application of nanosuspensions
Table no: 02 Applications of nanosuspension
|
SL NO: |
Route of administration |
Drugs |
Advantages |
|---|---|---|---|
|
01 |
Oral |
Atovaquone, Bupravaquone, NSAIDs. |
· Improved oral bioavailability and solubility in case of insoluble drugs. · Reduce the dosing frequency. · Large surface area leads to enhance the onset of action. · Minimize the peak plasma concentration (Cmax) in case of irritant drugs. |
|
02 |
Parenteral |
Anti-cancers |
· Reduce the toxicity. · Enhancing the permeability · Particle size of 100-300nm can be effectively targeting the tumor cells. |
|
03 |
Dermal |
Anti-oxidants like Resveratrol |
· Increasing the physical stability. |
|
04 |
Ocular |
Flurbiprofen and Ibuprofen |
· Rapid dissolution and bioavailability. · Sustained release if the proper surface modification can be done by using suitable bio adhesive hydrophilic polymer. · Eye irritation and blurred vision can be reduced |
|
05 |
Pulmonary |
Corticosteroids, Antiasthmatic agents |
· An aqueous nebulizer, could achieve pulmonary drug administration by producing a suitable droplet size. · It is easy to achieve the droplet size in range of 01-05 can spread them rapidly on the lung surface to enable better absorption. · If the particle size could be <0.5μm can easily exhale
|
Marketed Products
Table no: 03 Current marketed products of nanosuspension formulations
|
Sl no |
Drugs |
Product |
Method |
Company name |
Indication |
|
1 |
Fenofibrate |
Triglide |
Media milling |
Abbott pharma |
Hypercholesterolemia |
|
2 |
Fenofibrate |
Tricor |
HPH |
First Horizon Pharma |
Hypercholesterolemia |
|
3 |
Megestrol acetate |
Megace |
Media milling |
PAR Pharma |
Appetite stimulant |
|
4 |
Sirolimus |
Rapamune |
Media milling |
Wyeth |
Immunosuppressant |
|
5 |
Griseofulvin |
Gris-PEG |
Co-precipitation |
Novartis |
Antifungal |
|
6 |
Cyclosporine |
- |
HPH |
- |
Immunosuppressant |
|
7 |
Spironolactone |
- |
HPH |
- |
Diuretic |
|
8 |
Itraconazole |
- |
Precipitation |
- |
Antifungal |
|
9 |
Nabilone |
Cesamet |
Co-precipitation |
Lilly |
Anti-emetic |
|
10 |
Aprepitant |
Emend |
Media milling |
Merck Pharma |
Anti-emetic |
Conclusion : Nanosuspensions appear to be unique & yet commercially viable approach to combating problems such as poor bioavailability that are associated with the delivery of hydrophobic drugs, including those that are poorly soluble in aqueous as well as organic media. The dissolution problems of poorly water soluble drugs have been largely solved to improve drug absorption & bioavailability. Nanosuspension technology can be combined with traditional dosage forms: tablets, capsules, pellets, & can be used for parenteral products. To take advantages of nanosuspension drug delivery, simple formulation technologies & variety applications, nanosuspensions will continue to be interest as oral formulations & non-oral administration develop in the future.
References:
1) Shid RL, Dhole SN, Kulkarni N, Shid SL. A review on nanosuspension. Int. J. Pharm. Sci. Rev. Res 2013; 22(01):098-106.
2) Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian journal of pharmaceutical science 2015; 10(01):013-023.
3) Jassim ZE, Rajab NA. Review on preparation, characterization, and pharmaceutical application of nanosuspension as an approach of solubility and dissolution enhancement. Journal of pharmacy research 2018; 12(05):771-74.
4) Mane AN, Gilda SS, Ghadge AA, Bhosekar NR, Bhosale RR. Nanosuspension as a lipophilic drug carrier. Sch. Acad. J. Pharm 2014; 03(01)082-88.
5) Aher SS, Malsane ST, Saudagar BR. An over view on nanosuspension. Int J Cur Pharm Res 2017; 09(03):19-23.
6) Patel HM, Patel BB, Shah CN. Nanosuspension as a novel approach to enhance the solubility of poorly water soluble drugs. International Journal of Advances in Pharmaceutics 2016;5(2):021-25.
7) Vedaga SB, Gondkar SB, Saudagar RB. Nanosuspension as an emerging trend to improve the solubility of poorly water soluble drugs. Journal of Drug Delivery & Therapeutics 2019; 9(3):549-553.
8) Chandra A, Soni RK, Sharma U, Jain SK. Nanosuspension an overview. Journal of Drug Delivery & Therapeutics 2013; 3(6):162-67.
9) Wang L, Juan Du, Zhou Y, Wang Y. Safety of nanosuspensions in drug delivery. Nanomedicine: Nanotechnology, Biology, and Medicine 2017; 13:455–69.
10) Ahire E, Thakkar S, Darshanwad M, Misra M. Parenteral nanosuspensions: a brief review from solubility enhancement to more novel and specific applications. Acta Pharmaceutical Sinica B 2018; 8(5):733–55.
11) Dinesh kumar B, Nanosuspension Technology in Drug Delivery System, Nanoscience and Nanotechnology: An International Journal 2013; 3(1): 1-3.
12) Jorvekar.P, Pathak AA, Chaudhari PD. Formulation Development of Aceclofenac Loaded Nanosuspension by Three Square (32) Factorial Design, International Journal Of Pharmaceutical Sciences and Nanotechnology, 4, 2012, 1575-1582.
13) Kumar BS, Review Article Increasing Possibilities of Nanosuspension, Journal of Nanotechnology, 2013, 1-12.
14) Shid RL, Dhole SN, Kulkarni N, Shid SL. Nanosuspension: A review. Int J Pharm Sci Rev Res 2013; 22:98-106.
15) Chen A, Shi Y, Yan Z, Hao H, Zhang Y, Zhong J, et al. Dosage form developments of nanosuspension drug delivery system for oral administration route. Curr Pharm Design 2015; 21:4355 65.
16) Hater RC, Desai U, Chavan R. Review: Nanosuspensions. Int J Pharm Sci Rev Res 2012; 13:118-24.
17) Yadollahi R, Vasilev K, Simovic S. Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater 2015; 2015:1.
18) Geetha G, Poojitha U, Khan U. Various techniques for preparation of nanosuspension: A Review. Int J Pharm Res Rev 2014; 3:30-7.
19) Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, et al. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm 2015; 495:738-49.
20) Mathew M, Krishnakumar K, Dineshkumar B, Nair SK. Antibiotic’s nanosuspension: A review. J Drug Deliv Ther 2017; 7:128-31.
21) Mishra S. Nanosuspension in advanced drug delivery. Int J Adv Pharm Sci 2017; 1:56-65.
22) Malamatari M, Taylor KM, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov Today 2018; 23:534 47.
23) Mirza RM. A nanocrystal technology: To enhance solubility of poorly water soluble drugs. J Apply Pharm Res 2017; 5:1-13.
24) Narkhede K. A brief review on nano-pharmaceutical technology. J Pharm Sci 2015; 5:520-8.
25) Reimondez-Troitin˜o S et al. Nanotherapies for the treatment of ocular diseases. Eur J Pharm Biopharm.2015; 95: 279-293.
26) Debjit B, Harish G, Duraives S, Kumar K; Nanosuspension- A novel approaches in drug delivery systems, The Pharma Innovations, 2012; 1, 50-63.
27) Cornelia MK, Muller RH, Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization, Int. J. Pharm. Sci. Rev. Res, 2013; 22(1) : 20, 98-106
28) Chin WW, Parmentier J, Wyszynski M, Tan EH, Gokhale R. A brief literature and patent review of nanosuspension to a final drug product. J Pharm Sci 2014; 103:2980-99.
29) Cavalli FLR. Drug nanosuspensions a ZIP tool between traditional and innovative pharmaceutical formulations. Expert Opin Drug Deliv 2015; 12:1607-25.
30) Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, et al. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm 2015; 495:738-49.
31) Wang Y, Song J, Chow SF, Chow AHL, Zheng Y. Particle size tailoring of ursolic acid nanosuspensions for improved anticancer activity by controlled antisolvent
32) Jeffrey AK, Joan WL, Diwei H, SI K. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015; 10:2229-47
33) Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release 2014; 183:51-66.
34) Jain K, Mehra N, Jain N. Nanotechnology in drug delivery: safety and toxicity issues. Curr Pharm Des 2015; 21:4252-61.
35) Tuomela A, Liu P, Puranen J, Rönkkö S, Laaksonen T, Kalesnykas G, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm 2014; 467:34-41.
36) Guarnieri D, Sabella S, Muscetti O, Belli V, Malvindi MA, Fusco S, et al. Transport across the cell-membrane dictates nanoparticle fate and toxicity: a new paradigm in nanotoxicology. Nanoscale 2014; 6:10264-73.
37) Lamberti M, Zappavigna S, Sannolo N, Porto S, Caraglia M. Advantages and risks of nanotechnologies in cancer patients and occupationally exposed workers. Expert Opin Drug Deliv 2014; 11:1087-101.
38) Kanakia S, Toussaint JD, Chowdhury MS, Tembulkar T, Lee S, Jiang Y-P, et al. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials 2014; 35:7022-31.
39) He W, Lu Y, Qi J, Chen L, Hu F, Wu W. Food proteins as novel nanosuspension stabilizers for poorly water-soluble drugs. Int J Pharm 2013; 441:269-78.
40) Suchaoin W, Pereira de Sousa I, Netsomboon K, Lam HT, Laffleur F, Bernkop-Schnurch A. Development and in vitro evaluation of zeta potential changing self-emulsifying drug delivery systems for enhanced mucus permeation. Int J Pharm 2016; 510:255-62.
41) Burnea LC, Zaharescu T, Dumitru A, Plesa I, Ciuprina F. Radiation stability of polypropylene/lead zirconate composites. Radiat Phys Chem 2014; 94:156-60.
42) Varca GHC, Queiroz RG, Lugiao AB. Irradiation as an alternative route for protein crosslinking: cosolvent free BSA nanoparticles. Radiat Phys Chem 2016; 124:111-5.
43) Toh M-R, Chiu GNC. Liposomes as sterile preparations and limitations of sterilisation techniques in liposomal manufacturing. Asian J Pharm Sci 2013; 8:88-95.
44) Wei XL, Han YR, Quan LH, Liu CY, Liao YH. Oily nanosuspension for long-acting intramuscular delivery of curcumin di decanoate prodrug: preparation, characterization and in vivo evaluation. Eur J Pharm Sci 2013; 49:286-93.
45) da Silva SB, Ferreira D, Pintado M, Sarmento B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery – in vitro tests. Int J Biol Macromol 2016; 84:112-20.
46) Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics 2016; 8:16-50.
47) Shah CN. Nanosuspension: a novel approach to enhance solubility of poorly water soluble drugs. Pharma SciMonit 2016:7.
48) Tang X, LiangY, FengX, ZhangR, JinX, SunL. Co-delivery of docetaxel and Poloxamer235by PLGA–TPGS nanoparticles for breast cancer treatment. Mater Sci Eng C 2015; 49:348–55.
49) Chen X, Zhi F, Jia X, Zhang X, Ambardekar R, Meng Z, et al. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol 2013; 65:807–16.
50) Yap L-S, YangM-C Evaluation of hydrogel composing of Pluronic F127 and carboxy methyl hexanoyl chitosan as injectable safe fold for tissue engineering applications. Colloid Surf B 2016; 146:204–11.
51) Zhang W, Haman KJ, Metzger JM, Hackel BJ, Bates FS, Lodge TP. Quantifying binding of ethylene oxide–propylene oxide block copolymers with lipid bilayers. Langmuir 2017; 33:12624–34.
52) Liu P, Viitala T, Kartal-Hodzic A, Liang H, Laaksonen T, Hiroden J, et al. Interaction studies between indomethacin nanocrystals and PEO/PPO co-polymer stabilizers. Pharm Res 2015; 32:628–39.
53) Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release 2013; 172:1126–41. 60.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









