{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Bhupender Singh, Arun Nanda, Vikaas Budhwar, Rakesh K. Marwaha
Department of Pharmacy,
M. D. University, Rohatak
Haryana, India
* pharma.bsingh@gmail.com
ABSTRACT
Generic drugs are as effective as their branded counterparts in terms of safety and efficacy. Although their exists several myths about quality of generic medicines because of its less price as compared to branded counterparts. The present study aims to evaluate and compare the quality of generic medicine with their branded counterparts as per Indian Pharmacopoeial standards and other validated methods on a commonly used type 2 diabetes drug (Metformin). The qualitative as well as quantitative studies were performed as per IP 2010. The official test performed includes uniformity of weight, disintegration, dissolution, assay and friability. Non official test includes hardness test and assay by HPLC using validated methods. The study revealed that branded as well as generic metformin tablets comply with the standards provided in IP 2010 and generic metformin was found to be 111.52% lesser in cost per tablet as compared to costliest branded version of metformin.
REFERENCE ID: PHARMATUTOR-ART-2439
|
PharmaTutor (ISSN: 2347 - 7881) Volume 4, Issue 10 Received On: 03/05/2016; Accepted On: 23/05/2016; Published On: 01/10/2016 How to cite this article: Singh B, Nanda A, Budhwar V, Marwaha RK; A comparative evaluation of the quality & price of generic medicine with their branded counterparts; PharmaTutor; 2016; 4(10); 43-49 |
INTRODUCTION
A baguette brand bag costs more because of its good quality as compared to a non-branded bag, although it is not the same in the case of quality of low cost generics or non-branded medicines when compared to their branded counterparts. Generic medicines are equally effective and safe in terms of quality to their costly branded counterpart. A study reported by US FDA of the evaluation of the results of 38 published clinical trials that compared cardiovascular generic medicines to their branded counterparts revealed no evidence that brand-name heart drugs worked any better than generic heart drugs [1].
According to World Health Organization (W.H.O.) “a generic drug is a pharmaceutical product, usually intended to be interchangeable with an innovator product that is manufactured without a license from the innovator company and marketed after the expiry date of the patent or other exclusive rights” [2].
When it comes to prescribing a generic medicine most of the doctors in India and several other countries are found to be reluctant in prescribing generic medicine due to cynical about the quality, safety and efficacy of generic medicine. Medical Council of India, Code of EthicsRegulations regarding the use of Generic names of drugs says “Every physician should, as far as possible, prescribe drugs with generic names and he / she shall ensure that there is a rational prescription and use of drugs” [3]. Prescribing medicine with generic name was also recommended by Parliamentary Standing Committee (PSC) for Health and Family Welfare to the ‘Rajya Sabha’ on August 4, 2010 [4]. Several studiesconducted worldwide raise the issue of myths and prejudice regarding the ‘quality’ of generic medicine in the minds of patients, healthcare professionals and even the medical specialists.
A study conducted to evaluate perceptions of physician about generic medicine in USA in 2011 revealed that out of 506 physicians surveyed more than 23% of physicians expressed negative perceptions about efficacy of generic drugs, almost 50% reported negative perceptions about quality of generic medications and more than one quarter do not choose to use generics as first-line medications for themselves or for their families [5].
Similarly a study in Malaysia revealed that majority of physicians was positive about generics substitution but doubtfulabout their quality in terms of efficacy and safety for some drug categories [6]. According to a survey report conducted on 500 doctors in Haryana, India reveals that 40% of doctors in private as well as in public facilities never prescribe a generic medicine [7]. There are several similar studies worldwide by Jamshed et al. [8], Yousefi et al. [9], Kumar et al. [10] and Gupta et al. [11], which describe the misconception in minds of physician as well as patient about the quality of generic medicine.
A generic medicine costs less from that branded medicine because a brand name medicine has to go through 10-15 years of research and testing in animals and clinical trials in proving that it is safe and effective before it can be sold to the public. The testing can cost over USD 1 billion. Once the new drug is approved, the company that made and tested it files for patent protection and as it receives a patent, that company is only the sole manufacturer of that medicine. When a patent for a brand name medicine expires, any other company can copy the medicine and sell a generic version. These other companies are required to only prove that their product is the same as the brand name medicine through Bioavailability/Bioequivalence studies [12]. This means that generic drug companies do not have to spend as much time and money because they do not have to conduct clinical trials.Since there areample of companies manufacturing the generic medicine, due to competition the price is lowered even farther.
Skyrocketing cost of medicine has always been a matter of great concern to the health authorities providing health care to mankind. Efforts are made globally to provide quality medicine at affordable prices to the public at large. Generic medicines provide the same medical benefits to the suffering mankind as these are certified to be perfect substitute for the innovator branded product and its use can reduce the healthcare expenditure to a great extent.The effective use of generic products saved the U.S. health system nearly USD 1.5 trillion over the past 10 years (2004-2013) as per the IMS Institute for Healthcare Informatics [13]. As per US-FDA data nearly 8 in 10 prescriptions filled in the United States are for generic drugs (2014) [14].
In India due to the prejudice in the minds of physicians regarding the safety and efficacy of generics, their use is hindered and further as per law pharmacist cannot substitute the brand name with generic [15] so consumer has to buy costly branded medicine even though the equivalent low cost generic medicines are available.
With a view to adjudge and compare the quality of generic medicines with that of their costly branded counterparts, a comparative quality evaluation study was undertaken on commonly used drug for type 2 diabetes i.e. Metformin. India has more diabetics than any other country in the world, as per the International Diabetes Foundation the estimate of the actual number of diabetics in India is around 40 million (2014) [16]. So a commonly prescribed medicine (metformin) was selected and their existed remarkable price difference between generic and branded metformin.
MATIRIALS AND METHODS
Materials:
Standard Metformin was procured as a gift sample from Mankind Pharma. The three branded marketed formulation of Metformin was purchased from authorized dealer (Glycomet®, Gluformin® and Glyciphage®) and generic version was obtained from Jan Aushadhi store, Shastri Bhawan, New Delhi. All of the samples purchased were containing 500 mg of Metformin and all of them were in tablet dosage form. The efforts were made to procure these test samples having almost identical dates of manufacturing to rule out the possibility of difference in assay of the samples bearing different dates of manufacturing. The chemicals Acetonitrile, O-phosphoric acid were of HPLC grade and Sodium Hydroxide, Potassium di-hydrogen phosphate were of analytical grade. Milli-Q-Water was used for preparing all HPLC solutions and rest procedure distilled water was used.
Methods:
1.OFFICIAL TESTS
The following official tests were performed on all the three branded and generic metformin. Identification of Standard metformin was done as per IP 2010 through Infrared Spectroscopy. The IR spectrum of standard metformin is provided in Figure 1.3.
(i) Uniformity of weight: 20 tablets selected randomly weighed individually (using Mettler Toledo weighing balance) 20 units and average weight was calculated.
(ii) Dissolution: Dissolution study was performed using the IP Apparatus 2 (Lab India DS 8000-basket type) in 900 ml phosphate buffer pH 6.8, maintained at 37±0.5°C with paddle speed at 100 rpm for 45 minutes. 5 milliliters of samples were withdrawn at specified time intervals. The volume of dissolution fluid was adjusted to 900 ml, by replacing each 5 ml aliquot withdrawn with 5 ml of phosphate buffer pH 6.8, pre-warmed at 37±0.5°C. Samples withdrawn were filtered through whatmann filter paper (no.41), suitably diluted with phosphate buffer pH 6.8, and analyzed at 233 nm, using UV-Visible double beam spectrophotometer (UV-Visible spectrophometer 3000, Lab India). The content of C4H11N5.HCl was calculated in the medium taking 806 as the specific absorbance at 233 nm. The percentage drug release is depicted in Figure 1.1.
(iii) Disintegration Test: Disintegration was performed using basket rack assembly (IP 2010) maintained at 37±2°C. One tablet was introduced into each of 6 tubes and, a disc was added on each tube. The assembly was suspended in the beaker containing distilled water and operated for the specified time.
(iv) Assay: 20 tablets were weighed and powdered. The exact amount of powder was weighed containing about 0.1 g of Metformin Hydrochloride; it was shaken with 70 ml of distilled water for 15 minutes, diluted to 100.0 ml and filtered. 10.0 ml of this filtrate diluted to 100.0 ml with distilled water. Further diluted 10.0 ml of above solution to 100.0 ml with distilled water and measured the absorbance of the resulting solution at the maximum at about 232 nm. The content of C4H11N5.HCl was calculated taking 798 as the specific absorbance at 232 nm.
(v) Friability: A sample of whole tablets (11 tablets) corresponding to about 6.5 g weight was taken. The tablets were placed in drum and rotated it 100 times at 25 rpm. Tablets were removed and any loose dust from them also discarded and weighed. Dedusted the tablets carefully and weighed accurately.
2.UNOFFICIAL TESTS
The assay of metformin was also performed by HPLC through modified method [17].
(i) Assay: Accurately weighed 100 mg of standard metformin and dissolved in mobile phase; Acetonitrile:Water acidified to pH 3 by O.P.A (30:70). This solution was filtered through whatmann filter (No.41) and 7 dilutions were made and assay was performed using HPLC (Agilent 1200 series) by Agilent ODS column (250 mm x 4.6 mm i.d., 5 µm particle size). Flow rate of mobile phase was set to 1 ml/min and injection volume was 20 µl. The wavelength for detection used was 250 nm and column compartment temperature was 30ºc. The standard plot of Metformin by HPLC is depicted in Figure 1.2. Similarly the samples were examined in triplicate. 20 tablets were weighed individually and average weight was calculated and powdered. Exact amount of powder was weighed containing 100 mg of metformin and dilution was made in mobile phase, filtered and examined in same conditions as that of standard.
(ii) Hardness Test: Hardness was tested using Pfizer Hardness Tester. The tablet was placed between two platens and compressed and the value of the hardness is measured (kg/cm2). The platens should be parallel and their faces should be polished smooth and precision-ground perpendicularly to the direction of movement.
PRICE VARIATION OF BRANDED MEDICINE TO THAT OF GENERIC MEDICINE
Out of all the three branded metformin formulation from USV- Glycomet®, Abbott- Gluformin® and Franco Indian- Glyciphage® and one generic version from Jan Aushadhi contained the same labeled amount of metformin i.e. 500mg but the price per tablet varied. The per tablet price of Glycomet®, Gluformin® and Glyciphage® were Rs.1.69, Rs.1.63 and Rs.1.46 while that of generic was Rs.0.48 per tablet. This shows a price variation of 111.52% as compared to branded version. This data suggest that switching the prescription from branded to generic drug can result to a huge amount cost saving and cutting down the health care expenditure without compromising with the safety, quality and efficacy of medicinal product. The trade name, price, batch number, strength and manufacturer is depicted in Table 1 (b) and Figure 1.4 shows the percent variation in price of 4 variants.
RESULTS AND DISCUSSIONS
In this research work, the quality of generic Metformin (Jan Aushadhi Store) was compared to that of branded Metformin (Glycomet®, Gluformin®, and Glyciphage®), using pharmacopoeial and other tests. It was found that the generic Metformin was of “similar” pharmacopoeial standards, as that of branded Metformin, although the generic Metformin was much cheaper than the branded Metformin (111.52 % cheaper than costliest branded counterpart). The branded and generic variant showed comparable results. The assay was found to be more than 95% and less than 105% as per IP 2010, of all the branded (100.37%, 99.74%, 99.24%) and generic medicine (99.87%). (The results of the entire tests performed is depicted in Table 1 (a)) Hence, the general notion and doubt regarding the quality of the generic version of medicines needs to be expunged and government must campaign to promote generics about their quality and efficacy.
CONCLUSION
All the four variants of metformin tablets confirmed with the limits provided in IP 2010, in term of Uniformity of weight, Disintegration, Dissolution, Assay and Friability. This confirms that the generic medications are of equivalent and comparable quality of the costlier branded medicines available in the market. The government should therefore expand the scheme of opening Jan Aushadhi stores and promoting generics throughout the country to provide quality medicine at affordable prices to larger section of its population. There is also dire necessity of expanding the scope of the study to entire range of generic medicines available at different centers to eradicate the myth about quality and safety of generic medicines. Creating public awareness by advertisements in print as well as electronic media to instill confidence in the minds of patients and physician regarding quality and efficacy of such drugs and also to propagate that patient ask their doctors to prescribe medicines in generic name rather than the brand name /trade name through which the benefit of the scheme can be finally be availed.
Tables & Figures:
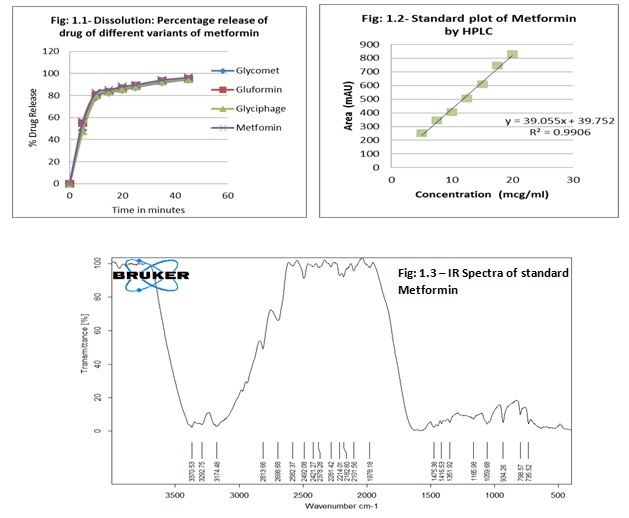
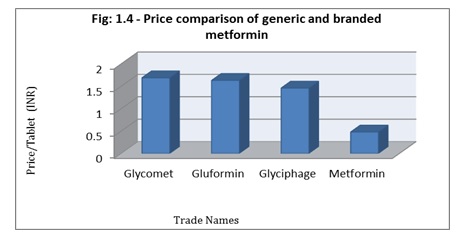
Table 1 (a): Results of tests performed.
|
TRADE NAME |
GLYCOMET |
GLUFORMIN |
GLYCIPHAGE |
GENERIC- METFORMIN |
||||||
|
TEST |
||||||||||
|
Uniformity of Weight |
Observations (Average Weight of 20 tablets in mg) |
586.15 |
596.02 |
549.54 |
628.94 |
|||||
|
Limits (As per I.P. 2010) |
556.75- 615.36 |
566.22- 625.82
|
522.06- 577.02
|
597.52-660.31 |
||||||
|
Results |
All the 20 tablets comply with the test and were found within the above range. |
|||||||||
|
Dissolution |
Observations (% Amount of Drug Release after 45 minutes) |
94.43% |
95.02% |
94.75% |
96.25% |
|||||
|
Limits (As per I.P. 2010) |
Not less than 70% of the label claimed |
|||||||||
|
Results |
Complies |
|||||||||
|
Disintegration |
Observations ( Average time of 6 tablets on minutes) |
12.11 |
4.25 |
3.38 |
3.32 |
|||||
|
Limits (As per I.P. 2010) |
Not more than 15 minutes |
|||||||||
|
Results |
Complies |
|||||||||
|
Assay |
Observations (% of drug content) |
100.37% |
99.74% |
99.24% |
99.87% |
|||||
|
Limits (As per I.P. 2010) |
Not less than 95% and not more than 105% |
|||||||||
|
Results |
Complies |
|||||||||
|
Friability |
Observations (% weight lost after friability) |
0.46 |
0.31 |
0.15 |
0.29 |
|||||
|
Limits (As per I.P. 2010) |
Not more than 1% |
|||||||||
|
Results |
Complies |
|||||||||
|
Hardness Test |
Observations |
10 kg/cm2 |
8 kg/cm2
|
7.5 kg/cm2
|
6.5 Kg/cm2 |
|||||
|
Limits |
5-10 Kg/cm2 |
|||||||||
|
Results |
Complies |
|||||||||
|
Assay by HPLC |
Observations (AUC) |
414.56 |
405.25 |
395.07 |
394.72 |
|||||
|
Limits (As per IP 2010) |
385.84 to 426.46 |
|||||||||
|
Results |
Complies |
|||||||||
Table: 1. (b) Branded and Generic Metformin.
|
Trade Name
|
Company |
IIN Name & Strength |
Price (Rupees) |
Packing |
Price/Tab (Rupees) |
|
GLYCOMET |
USV |
Metformin-500mg |
16.95 |
1*10 |
1.69 |
|
GLUFORMIN |
Abbott |
Metformin-500mg |
16.38 |
1*10 |
1.63 |
|
GLYCIPHAGE |
Franco-Indian Pharmaceuticals Ltd. |
Metformin-500mg |
29.20 |
1*20 |
1.46 |
|
METFORMIN |
Jan Aushadhi |
Metformin-500mg |
4.80 |
1*10 |
0.48 |
REFERENCES
1.Kesselheim, A. S., Misono, A. S., Lee, J. L., Stedman, M. R., Brookhart, M. A., Choudhry, N. K., & Shrank, W. H. (2008). Clinical Equivalence of Generic and Brand-Name Drugs Used in Cardiovascular Disease: A Systematic Review and Meta-analysis. JAMA : The Journal of the American Medical Association,300(21), 2514–2526.
2.WHO-Generic Definition (Retrieved from: http://www.who.int/trade/glossary/story 034/en/).
3.Medical Council of India, (http://www.mciindia.org/RulesandRegulations/CodeofMedicalEthics Regulations2002.aspx)
4.Ray Tapan, (http://www.tapanray.in/mci-asks-doctors-to-prescribe-drugs-in-generic-names/)
5.Shrank H. William, Liberman N. Joshua, Fischer A. Michael, Girdish Charmaine, Brennan A. Troyen, and Choudhry K. Niteesh: Physician Perceptions about Generic Drugs. The Annals of Pharmacotherapy, 2011.
6.Kumar Rohit, Hassali Azmi Ahmad Mohamed, Kaur Navneet, Kader Ali SK Muhamad: Perceptions of physicians from private medical centers in Malaysia about generic medicine usage: a qualitative study:Generics andBiosimilars Initiative Journal 2014.
7.Singal GL, Nanda A.: Evaluating general practitioners perceptions and practice on generic and branded medicines: A pilot study from the state of Haryana (India). The Pharma Review. 2010
8.Jamshed Qasim Shazia, Izham Mohamed, Ibrahim Mohamed, Hassali Azmi Ahmad Mohamed, Masood Imran, Low Yean Bee 4, Shafi Akmal Asrul and Babar Zaheer-ud-din. Perception and attitude of general practitioners regarding generic medicines in Karachi, Pakistan: A questionnaire based study.Southern Med Review 2012.
9.Yousefi Nazila, Mehralian Gholamhossein, Peiravian Farzad, Jahangiri Simindokht, Ahmadi Razieh. Physicians’ perceptions of generic medicine in Iran. Drugs & Therapy Perspectives July 2015.
10.Kumar Rohit, Hassali Azmi Mohamed, Saleem Fahad, Alrasheedy A. Alian, Kaur Navneet, Wongand Y. Zhiand Kader Ali SK Abdul Muhamad. Knowledge and perceptions of physicians from private medical centers towards generic medicines: A Nationwide Survey from Malaysia. Journal of Pharmaceutical Policy and Practice 2015.
11.Gupta SK, Nayak RP, Vidyarthi SK. A study on the knowledge, attitude, and the practice of generic medicines among the doctors in a tertiary care teaching hospital in South India. National Journal Physiology, Pharmacy and Pharmacology.2015.
12.Hupila K. Mathew & Smith L. Dorothy: 5 Common Questions about Generic Drugs Consumer Health Information Corporation, 2010. (Retrieved from:http://www.consumer-health.com/services/5CommonQuestionsAboutGeneric Drugs.php).
13.IMS- Generic Drug Savings in US, GPhA-2014 (Retrieved from: http://www.gphaonline.org/media/cms/GPhA_Savings_Report.9.10.14FINAL.pdf).
14.FDA- Facts about Generic Drugs. (Retrieved from: http://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandinggenericdrugs/ucm167991.htm).
15.Drugs and Cosmetics Rules 1945,(Retrieved from: http://www.naco.gov.in/upload/2014%20mslns/BTS/Drug%20&%20Cosmetic%20Act%201940.pdf)
16.Gale Jason (November 7, 2010). "India’s Diabetes Epidemic Cuts Down Millions Who Escape Poverty". Bloomberg. Retrieved from: http://www.bloomberg.com/news/articles/2010-11-07/india-s-deadly-diabetes-scourge-cuts-down-millions-rising-to-middle-class.
17.Bhamare P.C., Bari S.B., Natarajan S., Patil A.A., Patil S.H., and Shirode P.T. Development and Validation of a precise single stability indicating HPLC method for determinations of Metformin hydrochloride and Fenofibrate, in Pure form and in Pharmaceutical tablets, International Journal of Pharmaceutical Technology and Research: 2011.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









