Q.4. (b) Explain Index of hydrogen deficiency and its significance
Ans.4.(b) Index of Hydrogen Deficiency
[adsense:336x280:8701650588]
The ion has to be an odd-electron ion, because the molecular ion is produced by loss of one electron from the neutral molecule, the converse however, is not true since there may well be odd-electron ions other than the molecular ion in the spectrum, arising from rearrangement reactions. When the elemental composition of the ion can be determined, the index of hydrogen deficiency (the sum of multiple bonds and ring systems) may be used to know if the ion is an odd-electron ion. The index of hydrogen deficiency is the no. of pairs of hydrogen atoms which must be removed from the saturated open-chain formula (e.g., CnH2n+2for alkanes) to give the observed molecular formula. The index of hydrogen deficiency is then the sum of the no. of rings, the no. of double bonds and twice the no. of triple bonds. For a molecule Iy IIn IIIz IVx, the index of hydrogen deficiency = x- y/2 + z/2 + 1
Where I = any monovalent atom
II = O,S or any other divalent atom
III = N,P or any other trivalent atom
IV = C,Si or any other tetravalent atom
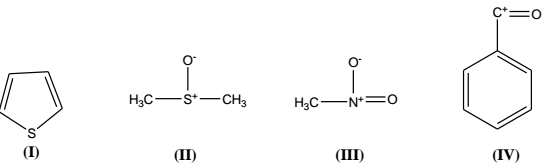
Thus, e.g., thiophen, C4H4S (I) has an index of hydrogen deficiency of (4- 4/2 + 1) = 3. When one compares C6H6 with C6H14, the index is 4 for the former and 0 for the later. In the consideration of the index, polar structures are used, thus the calculated index, 0 for dimethyl sulfoxide (DMSO) is in agreement with the structure II in which the Lewis octet rule is being obeyed. Similarly in nitromethane CH3NO2, the calculated index is 1, and this is in agreement with structure III. Thus any index of hydrogen deficiency greater than 1 may be due to a combination of these structural variations, i.e., an IHD of 2 will indicate 2 double bonds, 2 rings, 1 double bond and one ring or one triple bond. The index of hydrogen deficiency must be a whole no. for an odd electron ion, whereas for an even electron ion the value will be non-integral. Thus the values of index can be applied to the molecular ion as well as to the fragment ions to give useful information. Thus, for example, C7H5O+with an index of 5.5, points to IV as the reasonable assignment.