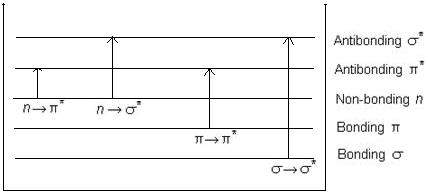
It was earlier stated that σ, π, and n electrons are present in molecule and can be excited from the ground state to excited state by the absorption of UV radiation. The various transitions are n→∏*, ∏→∏*, n→σ*, & σ →σ*
Fig 1: Energy levels of electronic transitions
Fig. 1 shows the energy requirements for different electronic transitions.
The energy requirement order for excitation for different transitions is as follows.
n→∏*< ∏→∏*< n→σ*< σ→σ*
n→∏* transition requires lowest energy while σ→σ* requires highest amount of energy.
[adsense:336x280:8701650588]
1. n→∏* transition
n→π* transition requires lowest energy due to longer wavelength. So they are forbidden and corresponding bands are characterized by low molar absorptivity. εmax < 100. It is also known as R- band. They are further characterized by hypsochromic shift or blue shift observed with an increase in solvent polarity.
2. ∏→∏* transition
It is due to the promotion of an electron from a bonding π orbital to an anti-bonding ∏* orbital. Energy requirement is between n→ ∏* and n→σ*. But the extended conjugation and alkyl substituents shifts the λmax towards longer wavelength (Bathochromic shift). It is also called K band.
3. n→σ* transition
Saturated compounds with lone pair of electrons undergo n→σ* transition in addition to σ→σ* transition. Corresponding absorption bands appear at longer wavelengths in near UV region.
4. σ→σ* transition
These transitions can occur in such compounds in which all the electrons are involved in single bonds and there are no lone pair of electrons.
Energy required for σ→σ* transition is very large so the absorption band occurs in the far UV region. So this transition cant normally be observed.
FIND MORE :-
| Theory of Spectroscopy | EMR Spectra |
| Beer-Lambert's Law | Instrumentation |
| Effect of Solvent | Applications |