{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Shashank Nayak N*1, U Srinivasa2, Shwetha S Kamath K3, Shabaraya AR4
1Dept of Pharmaceutics, Pacific university, Udaipur, Rajasthan
2Dept.of Pharmacognosy, Srinivas college of pharmacy, Mangalore, Karnataka
3Dept. of Pharmaceutics, Srinivas college of pharmacy, Mangalore, Karnataka
4Principal, Srinivas College of Pharmacy, Mangalore, Karnataka
shashanknayak87@gmail.com
ABSTRACT
In this current work simultaneous estimation of Moxifloxacin HCL and Ketorolac tromethamine in simulated tear fluid was performed by using UV spectroscopy. The zero order derivative spectroscopy revealed that Moxifloxacin HCL had a λ max of 288 nm in Simulated tear fluid (STF) and Ketotorolac tromethamine had λ max of 322nm respectively. The linearity was found in the range of 2-10 µg/ml for Moxifloxacin HCL and 2-14µg/ml for ketorolac tromethamine. The R2 value was found to be 0.998 and slope y=0.089 for Moxifloxacin HCL and R2 =0.998 and slope y=0.051 for Ketorolac tromethamine. The zero order spectrum was deravatized to first order derivative spectra for both the above mentioned drug. The first order derivative spectra of Moxifloxacin HCL and ketorolac tromethamine indicated that it had a zero crossing point (ZCP) at 286 nm and 328 nm. The absorbance of the mixture containing MOXI and KETO was found out at 286 nm and 328 nm which showed linearity and obeyed beers lamberts law.
INTRODUCTION
Moxifloxacin HCL is chemically 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)–octahydro-6H-pyrrolol[3,4-b]pyridine-6-yl]-4-oxo-3-quinolinecarboxylic acid mono hydrochloride. It comes under the fourth generation fluoroquinolone antibiotic. Moxifloxacin is a broad spectrum antibiotic which is active against both Gram-positive and Gram negative bacteria. The bactericidal action of moxifloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division[1-3].
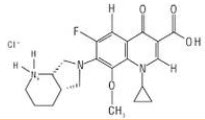
Figure 1:Structure of Moxifloxacin HCL
Ketorolac tromethamine is chemically 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid. Ketorolac is a nonsteroidal anti-inflammatory drug (NSAID) chemically related to indomethacin and tolmetin. Ketorolac tromethamine is a racemic mixture [+]R-enantiomeric forms, with the S-form having analgesic activity. Its anti inflammatory effects are believed to be due to inhibition of both cylooxygenase-1 cylooxygenase-2 (COX-2) which leads to the inhibition of prostaglandin synthesis leading to decreased formation of precursors of prostaglandins and thromboxanes arachidonic acid[4].
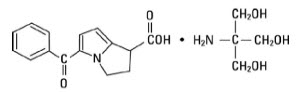
Figure 2: Structure of ketorolac tromethamine
Derivative spectrophotometry is an analytical technique of great utility for extracting both qualitative and quantitative information from spectra composed of unresolved bands. Although it was introduced more than thirty years ago, and has demonstrable advantages for the solution of specific analytical problems, this technique has been accepted only hesitantly, because of the initial lack of reasonably priced instrumentation and original limitation to the first derivative. However, in recent years, the introduction of electronic differentiation by a microcomputer interfaced with the spectrophotometer makes possible the plotting of the first, second or higher order derivatives of a spectrum with respect to wavelength[5].
In this current study simultaneous estimation of Moxifloxacin HCL and Ketorolac tromethamine was done in simulated tear fluid by using using zero order and first order derivative spectroscopic methods.
MATERIALS AND METHOD [6-13]
Instruments
UV-Visible double beam spectrophotometer ( SHIMADZU Co, Japan) with 1cm matched quartz cells, Micropipette of Variable volume 10-1000 μL (Gene Pete Co.) and Digital balance were used.
Materials
Moxifloxacin HCL was obtained as gift sample from micro labs Bangalore and Ketorolac tromethamine was obtained as gift sample from Ranbaxy goa. All other reagents were of analytical grade.
Preparation of simulated tear fluid (STF)
The simulated tear fluid was prepared by taking the following ingredients
Sodium chloride-3.35 gms
Sodium bicarbonate-1.0 gms
Calcium chloride-0.04 gms
Distilled water-500 ml
Preparation of stock solution of Moxifloxacin HCL and Ketorolac tromethamine in STF
100 mg of Moxifloxacin HCL and Ketorolac tromethamine was dissolved seperately in 50 ml of Simulated tear fluid (Stock A). From stock A take 1 ml diluted to 100 ml of simulated tear fluid (stock B).
Preparation of working standard of Moxifloxacin HCL and ketorolac tromethamine
From stock B of both Moxifloxacin HCL and Ketorolac tromethamine,(2-10µg/ml) working standard solution was prepared for Moxifloxacin HCL and (2-14µg/ml) working standard was prepared for Ketorolac tromethamine.
Determination of λmax of Moxifloxacin HCL and construction of calibration curve in STF (zero order derivative spectra)
Wavelength scan was done from 200-400 nm by using 2-10 µg/ml solution in double beam UV spectrophotometer Shimadzu (UV-1601) to get λmax and absorbance of the standard solution were noted down.
Determination of λmax of Ketorolac tromethamine and construction of calibration curve in STF (Zero order derivative spectra)
Wavelength scan was done from 200-400 nm by using 2-14 µg/ml solution in double beam UV spectrophotometer Shimadzu (UV-1601) to get λmax and absorbance of the standard solution were noted down.
First order derivative spectra of Moxifloxacin HCL in STF
The zero order spectrum of Moxifloxacin HCL was deravatized to first order spectrum in the software and ZCP of Moxifloxacin HCL and absorbance at ZCP of 2-10µg/ml was noted down.
First order derivative spectra of Ketorolac tromethamine in STF
The zero order spectrum of Ketorolac tromethamine was deravatized to first order spectrum in the software and ZCP of Ketorolac tromethamine and absorbance at ZCP of 2-14µg/ml was noted down.
Determination of absorbance of Moxifloxacin HCL and Ketorolace tromethamine in mixture at ZCP
The ZCP of Moxifloxacin HCL was used as absorbance maxima for Ketorolac tromethamine and ZCP of Ketorolac tromethamine was used as absorbance maxima for Moxifloxacin HCL and absorbance was noted down and linearity graphs were plotted.
RESULTS AND DISCUSSION
λ max of Moxifloxacin HCL and calibration curve in STF (zero order derivative spectra)
The λ max of Moxifloxacin HCL was found out at 288 nm(Figure 3)and linearity was in the range of 2-10 µg/ml and R2 value was found to be 0.998, slope y=0.089. The absorbance was found at 288 nm is mentioned in figure no 4.
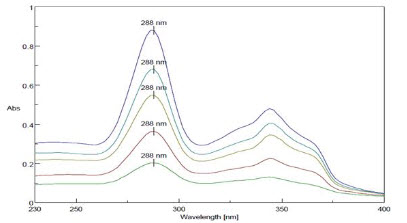
Figure 3-Overlay of λ max of Moxifloxacin HCL (2-10 µg/ml)
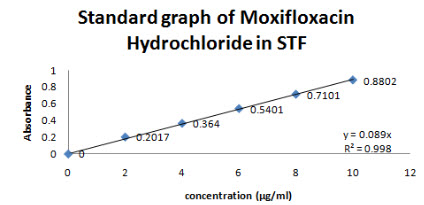
Figure 4-Standard graph of Moxifloxacin HCL in STF at 288nm
λ max of Ketorolac tromethamine and calibration curve in STF (zero order derivative spectra)
The λ max of Ketorolac tromethamine was found out at 322 nm(Figure 5)and linearity was in the range of 2-14 µg/ml and R2 value was found to be 0.998, slope y=0.051.The absorbance was found at 322 nm is mentioned in figure no 6.
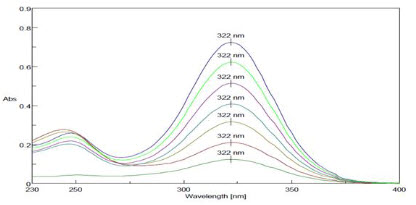
Figure 5-Overlay of λ max of ketorolac tromethamine (2-14 µg/ml)
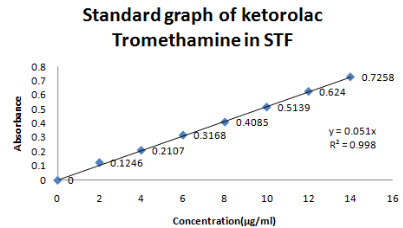
Figure 6-Standard graph of Ketorolac tromethamine in STF at 322nm
First order derivative spectra of Moxifloxacin HCL in STF
The zero order spectra of Moxifloxacin HCL which had λmax of 288 nm was deravatized in software to first order spectra. The ZCP was found at 286nm(Figure 7), so at this wavelength Moxifloxacin HCL showed almost zero absorbance(Figure 8) and R2 was found to be 0.999 and slope y= -0.001x and this wavelength was selected for checking the absorbance of Ketorolac tromethamine in mixture.
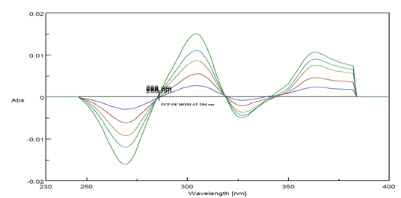
Figure 7-ZCP of Moxifloxacin HCL at 286 nm
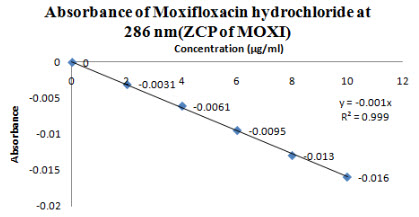
Figure 8-Absorbance of Moxifloxacin HCL at 286 nm(ZCP)
First order derivative spectra of Ketorolac tromethamine in STF
The zero order spectra of Ketorolac tromethamine which had λmax of 322 nm was deravatized in software to first order spectra. The ZCP was found at 328nm(Figure 9), so at this wavelength Ketorolac tromethamine showed almost zero absorbance(Figure 10) and R2 was found to be 0.998 and slope y= -0.001x and this wavelength was selected for checking the absorbance of Moxifloxacin HCL in mixture.
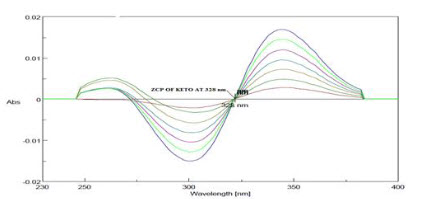
Figure 9-ZCP of Ketorolac tromethamine at 322 nm
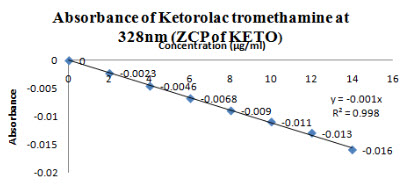
Figure 10-Absorbance of Ketorolac tromethamine at 328 nm(ZCP)
Determination of absorbance of Mixture of Moxifloxacin HCL and Ketorolac tromethamine at ZCP
100 mg of Moxifloxacin HCL and Ketorolac tromethamine was dissolved in 50 ml of STF(Stock A).From this stock solution 1 ml was taken and diluted to 100 ml of STF(Stock B). From this stock B (2-14 µg/ml) solution was prepared and absorbance was noted down at 328 nm for Moxifloxacin HCL(Figure 11) and 286 nm for Ketorolac tromethamine (Figure 12)
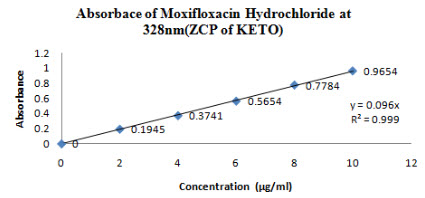
Figure 11-Absorbance of Moxifloxacin HCL at 328 nm(ZCP of Keto)
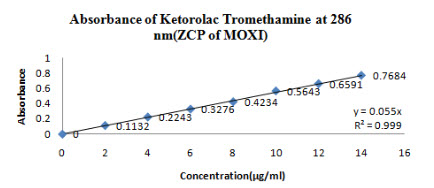
Figure 12-Absorbance of Ketorolac tromethamine at 286 nm(ZCP of Moxi)
CONCLUSION
In this current work first order derivative spectroscopy was used for analyzing the sample containing two drugs by considering the ZCP of individual drugs. So derivative spectroscopy can be used as best tool for analyzing Moxifloxacin HCL and Ketorolac tromethamine in mixture and formulation.
ACKNOWLEDGEMENT: All the authors are thankful to Srinivas college of pharmacy for providing the necessary facility to conduct the research work.
REFERENCES
1. drugs.com/monograph/moxifloxacin-hydrochloride.html
2. drugbank.ca/system/fda_labels/DB00218.pdf1265922809
3. Fatima V, Maria J. Pratas de Melo, Ana I. Cacuo, Ralf Dohrn, Foteini A. Makrydaki, Epaminondas Voutsas et al,. Solubility of Antibiotics in Different Solvents. 1. Hydrochloride Forms of Tetracycline, Moxifloxacin, and Ciprofloxacin. Ind Eng Chem Res. 2006; 45; 6368-74.
4. drugbank.ca/system/fda_labels/DB00465
5. F sanchez rojas, Bosch ojeda, j m cano pavon. Derivative ultraviolet-visible region absorption spectrophotometry and its analytical applications. :Telanta 1988; 35(10); 753-761
6. Shashank Nayak N, Bharani s sogali , R S thakur. Formulation and evaluation of ph triggered in situ ophthalmic gel of moxifloxacin hydrochloride. Int J of Pharm and Pharmaceutical sci 2012; 4(2):452-59
7. DR Mevada, K Bhalodiya, B Maniar, K Dadhania, S Faldu; Development and Validation of First Order Derivative Spectrophotometric Method for Simultaneous Estimation of Bromhexine Hydrochloride and Phenylephrine Hydrochloride in their Combined Pharmaceutical Dosage Form; Pharma Tutor; 2014; 2(6); 132-138
8. Fazil khan, Bm gurupadayya, Am nasefa, sai prudhvi. Development and validation of zero and first order spectrophotometric method for determination of opipramol in bulk and pharmaceutical dosage form. Int j pharm pharm sci.2015; 7(3); 274-277
9. Zamir G. Khan, Amod S. Patil, and Atul A. Shirkhedkar. Estimation of Tadalafil Using Derivative Spectrophotometry in Bulk Material and in Pharmaceutical Formulation. Int J of Spectroscopy:2014 (Article ID 392421 ):1-6.
10. Deep P. Patel, Ragin R. Shah, Alisha P. Patel, Ponal K. Tank. Development and validation of first order derivative uv-spectroscopic method for estimation of ibuprofen and famotidine in synthetic mixture. Pharma sci Monitor: 2012; 3(4); 2506-2515.
11. Ajit K. Nangare, Karan K. Pawa , Abhishek K. Shinde. Zero and first order derivative UV spectrophotometric methods for determination of efavirenz in pharmaceutical formulation. Der Pharmacia Lettre, 2014; 6(4);143-150.
12. Khutle Nilesh M, Pawar Harshal A and Vijaya C. “Zero-Crossing” First-order Derivative Spectroscopy Method for Analysis of Sertraline Hydrochloride in Novel Self Micro Emulsifying Drug Delivery System. Austin J Anal Pharm Chem. 2014; 1(3); 1013.
13. Dr. Atul patel. Development and validation of derivative spectoscopic method for simultaneous determination of nebivolol hydrochloride and s-amlodipine besylate in combined dosage form. Int J Pharm Bio sci; 2013; 4(3); 379 – 391.
REFERENCE ID: PHARMATUTOR-ART-2402
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 4 Received On: 01/11/2015; Accepted On: 07/11/2015; Published On: 01/04/2016 How to cite this article: Shashank NN, Srinivasa U, Kamath SS, Shabaraya AR; Zero order and First order derivative spectroscopic method for simultaneous estimation of Moxifloxacin hydrochloride and Ketorolac tromethamine in simulated tear fluid; PharmaTutor; 2016; 4(4); 43-47 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









