About Author:
Dheeraj Fulara
DIT Faculty of Pharmacy, Mussoorie Diversion Road, Dehradun 248009,
Uttarakhand, India.
fularadheerajmpharm@yahoo.com
Abstract-
Transdermal drug delivery systems (TDDS) are dosage forms involves drug transport to viable epidermal and or dermal tissues of the skin for local therapeutic effect while a very major fraction of drug is transported into the systemic blood circulation. The adhesive of the transdermal drug delivery system is critical to the safety, efficacy and quality of the product. Topical administration of therapeutic agents offers many advantages over conventional oral and invasive methods of drug delivery. Several important advantages of transdermal drug delivery are limitation of hepatic first pass metabolism, enhancement of therapeutic efficiency and maintenance of steady plasma level of the drug. This review article provides an overview of TDDS, its advantages over conventional dosage forms, drug delivery routes across human skin, penetration enhancers, various components of Transdermal patches, types of Transdermal patches, methods of preparation and its physicochemical methods of evaluation.
REFERENCE ID: PHARMATUTOR-ART-2025
INTRODUCTION
At present, the most common form of delivery of drugs is the oral route in the past, the most commonly applied systems were topically applied creams and ointments for dermatological disorders. The occurrence of systemic side-effects with some of these formulations is indicative of absorption through the skin. A number of drugs have been applied to the skin for systemic treatment. Transdermal delivery of drugs through the skin to the systemic circulation provides a convenient route of administration for a variety of clinical indications. Transdermal delivery systems are currently available containing scopolamine (hyoscine) for motion sickness, clonidine and nitroglycerin for cardiovascular disease, fentanyl for chronic pain, nicotine to aid smoking cessation,oestradiol (alone or in combination with levonorgestrel ornorethisterone) for hormone replacement and testosterone for hypogonadism. Despite the small number of drugs currently delivered via this route, it is estimated that worldwide market revenues for transdermal products are USD 3B, shared between the USA at 56%, Europe at 32% and Japan at 7%. In a recent market report it was suggested that the growth rate for transdermal delivery systems will increase 12%annually through to 2007 [1]. Transdermal products for cardiovascular disease, Parkinson’s disease, Alzheimer’sdisease, depression, anxiety, attention deficit hyperactivity disorder (ADHD), skin cancer, female sexual disfunction, post-menopausal bone loss, and urinary incontinence are at various stages of formulation and clinical development. Theapplication of transdermal delivery to a wider range of drugs is limited due to the significant barrier to penetration acrossthe skin which is associated primarily with the outer most stratum corneum layer of the epidermis. Consequently thedaily dose of drug that can be delivered from a Transdermalpatch is 5-10 mg, effectively limiting this route ofadministration to potent drugs. Significant effort has beendevoted to developing strategies to overcome the impermeability of intact human skin. These strategiesinclude passive and active penetration enhancement and technologies to bypass the stratum corneum. This reviewdescribes the routes of penetration, how drug properties influence penetration and the techniques that have been used to enhance penetration across human skin. Physical enhancement technologies such as iontophoresis, electroporation, phonophoresis, microneedles and jet injectors are reviewed in a separate article in this journal by Cross and Roberts [2] and in other recent review articles [3,4].
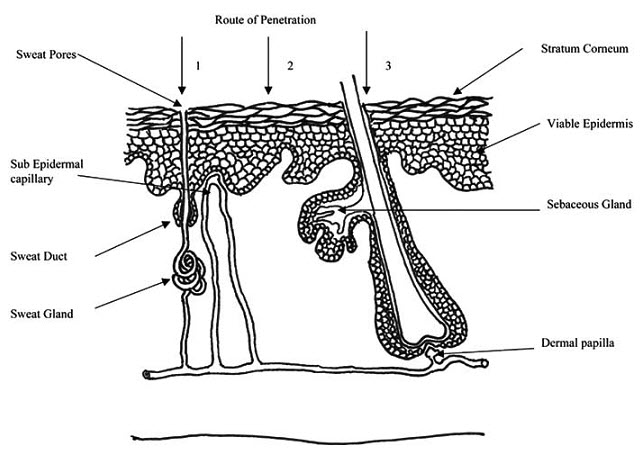
Fig 1-Simplified representation of skin showing routes of penetration:
1. through the sweat ducts; 2. directly across the stratum corneum; 3. via the hair follicles.
DEFINITION
Transdermal drug delivery systems are topically administered medicaments in the form of patches that deliver drugs for systemic effects at a predetermined and controlled rate.(1) Transdermal drug delivery has made an important contribution to medical practice, but has yet to fully achieve its potential as an alternative to oral delivery and hypodermic injections.
First-generation Transdermal delivery systemshave continued their steady increase in clinical use for delivery of small, lipophilic, low-dose drugs.
Second-generation delivery systems using chemical enhancers, noncavitational ultrasound and iontophoresis have also resulted in clinical products; the ability of iontophoresis to control delivery rates in real time provides added functionality.
Third-generation delivery systems target their effects to skin's barrier layer of stratum corneum using microneedles, thermal ablation, microderm abrasion, electroporation and cavitational ultrasound. Microneedles and thermal ablation are currently progressing through clinical trials for delivery of macromolecules and vaccines, such as insulin, parathyroid hormone and influenza vaccine. Using these novel second- and third-generation enhancement strategies, Transdermal delivery is poised to significantly increase its impact on medicine.
Interest in Transdermal has increased on several fronts over the past several years technology companies have generated additional clinical data demonstrating the potential advanced Transdermal technology, pharmaceutical companies there become more aggressive in exploring alternate formulations to extend patent.
ADVANTAGES AND DISADVANTAGES OF TRANSDERMAL DRUG DELIVERY (13)
Transdermal drug delivery systems offer several important advantages over more traditional approaches, including:
1 Longer duration of action resulting in a reduction in dosing frequency.
2 Increased convenience to administer drugs which would otherwise require frequent dosing.
3 Improved bioavailability.
4 More uniform plasma levels.
5 Reduced side effects and improved therapy due to maintenance of plasma levels up to the end of the dosing interval.
6 Flexibility of terminating the drug administration by simply removing the patch from the skin.
7 Improved patient compliance and comfort via non-invasive, painless and simple application.
Some of the greatest disadvantages to Transdermal drug delivery are:
1. Possibility that a local irritation at the site of application.
2. Erythema, itching, and local edema can be caused by the drug, the adhesive, or other excipients in the patch formulation.
3. Patches after defined dosage period.
4. Limited number of drugs is used by transdermal system.
PROPERTIES THAT INFLUENCE TRANSDERMAL DELIVERY(14)
1. Release of the medicament from the vehicle.
2. Penetration through the skin barrier.
3. Activation of the pharmacological response.
KINETICS OF TRANSDERMAL PERMEATION(15)
Knowledge of skin permeation kinetics is vital to the successful development of Transdermal therapeutic systems. Transdermal permeation of a drug involves the following steps:
1. Sorption by stratum corneum.
2. Penetration of drug through viable epidermis.
3. Uptake of the drug by the capillary network in the dermal papillary layer.
This permeation can be possible only if the drug possesses certain physiochemical properties.
The rate of permeation across the skin is given by
dQ
------ = Ps (Cd – Cr) ... …………(1)
dt
where Cd and Cr are the concentration of the skin penetrant in the door compartment i.e. on the surface of stratum corneum and in the receptor compartment i.e. body respectively. Ps is the overall permeability coefficient of the skin tissue to the penetrant. This permeability coefficient is given by the relationship:
DssKs
Ps = ----------
hs
where Ks is the partition coefficient for the interfacial partitioning of the penetrant molecule from a solution medium or a Transdermal therapeutic system on to the stratum corneum, Dss is the apparent diffusivity for the steady state diffusion of the penetrant molecule through a thickness of skin tissues and hs is the overall thickness of skin tissues. As Ks, Dss and hs are constant under given conditions permeability coefficient Ps for a skin penetrant can be considered to be constant. From equation (1) it is clear that a constant rate of drug permeation can be obtained only when Cd >> Cr i.e. the drug concentration at the surface of the stratum corneum Cd is consistently and substantially greater than the drug concentration in the body Cr. The equation becomes
dQ
------- = Ps Cd
dt
And the rate of skin permeation is constant provided the magnitude of Cd remains fairly constant throughout the course of skin permeation. For keeping Cd constant the drug should be released from the device at a rate Rr i.e. either constant or greater than the rate of skin uptake Ra i.e. Rr >> Ra. Since Rr >> Ra, the drug concentration on the skin surface Cd is maintained at a level equal to or greater than the equilibrium solubility of the drug in the stratum corneum Cs i.e. Cd >> Cs. Therefore a maximum rate of skin permeation is obtained and is given by the equation:
(dQ/dt)m = PsCs
From the above equation it can be seen that the maximum rate of skin permeation depends upon the skin permeability coefficient PS and equilibrium solubility in the stratum corneum CS. Thus skin permeation appears to be stratum corneum limited.
BASIC COMPONENTS OF TRANSDERMAL DRUG DELIVERY SYSTEMS (15)
The components of Transdermal devices include:
1. Polymer matrix or matrices
2. The drug
3. Permeation enhancers
4. Other excipients
1. Polymer Matrix
The Polymer controls the release of the drug from the device.
Possible useful polymers for Transdermal devices are:
a)Natural Polymers: E.g. Cellulose derivatives, Zein, Gelatin, Shellac, Waxes, Proteins, Gums and their derivatives, Natural rubber, Starch etc.
b) Synthetic Elastomers: E.g.Polybutadieine, Hydrin rubber, Polysiloxane, Silicone rubber, Nitrile, Acrylonitrile, Butyl rubber, Styrenebutadieine rubber, Neoprene etc.
c)Synthetic Polymers: E.g. Polyvinyl alcohol, Polyvinyl chloride, Polyethylene, Polypropylene, Polyacrylate, Polyamide, Polyurea, Polyvinylpyrrolidone, Polymethylmethacrylate, Epoxy etc.
2. Drug
For successfully developing a Transdermal drug delivery system, the drug should be chosen with great care. The following are some of the desirable properties of a drug Transdermaly delivery.
Physicochemical properties:
1. The drug should have a molecular weight less than approximately 1000 daltons.
2. The drug should have affinity for both – lipophilic and hydrophilic phases. Extreme partitioning characteristics are not conducive to successful drug delivery via the skin.
3. The drug should have low melting point.
Along with these properties the drug should be potent, having short half life and be none irritating.
3. Permeation Enhancers
These are compounds which promote skin permeability by altering the skin as a barrier to the flux of a desired penetrant.
These may conveniently be classified under the following main headings:-
a) Solvents
These compounds increase penetration possibly by swallowing the polar pathway and/or by fluidizing lipids. Examples include water alcohols – methanol and ethanol; alkyl methyl sulfoxides – dimethyl sulfoxide, alkyl homologs of methyl sulfoxide dimethyl acetamide and dimethyl formamide; pyrrolidone – 2 pyrrolidone, N-methyl, 2-purrolidone; laurocapram (Azone), miscellaneous solvents – propylene glycol, glycerol, silicone fluids, isopropyl palmitate.
b) Surfactants
These compounds are proposed to enhance polar pathway transport, especially of hydrophilic drugs. The ability of a surfactant to alter penetration is a function of the polar head group and the hydrocarbon chain length.
Anionic Surfactants: e.g. Dioctyl sulphosuccinate, Sodium lauryl sulphate, Decodecylmethyl sulphoxide etc.
Nonionic Surfactants: e.g. PluronicF127, Pluronic F68, etc
Bile Salts:e.g. Sodium taurocholate, Sodium deoxycholate, Sodium tauroglycocholate.
Binary System:e.g. these systems apparently open up the heterogeneous multi-laminate pathway as well as the continuous pathways. E.g. Propylene glycol-oleic acid and 1, 4-butane diol-linoleic acid.
c) Miscellaneous chemicals
These include urea, a hydrating and keratolytic agent; N, N-dimetyl-m-touamide; calcium thioglycolate; anticholinergic agents.
Some potential permeation enhancers have recently been described but the available data on their effectiveness sparse. These include eucalyptol, di-o-methyl-ß-cyclodextrin and soyabean casein.
4. Other Excipients
a) Adhesives:
The fastening of all Transdermal devices to the skin has so far been done by using a pressure sensitive adhesive which can be positioned on the face of the device or in the back of the device and extending peripherally. Both adhesive systems should fulfill the following criteria:
1- Should adhere to the skin aggressively, should be easily removed.
2- Should not leave an unwashable residue on the skin.
3- Should not irritate or sensitize the skin.
The face adhesive system should also fulfill the following criteria:
1-Physical and chemical compatibility with the drug, excipients and enhancers of the device of which it is a part.
2- Permeation of drug should not be affected.
3- The delivery of simple or blended permeation enhancers should not be affected.
b) Backing membrane:
Backing membranes are flexible and they provide a good bond to the drug reservoir, prevent drug from leaving the dosage form through the top, and accept printing. It is impermeable substance that protects the product during use on the skin e.g. metallic plastic laminate, plastic backing with absorbent pad and occlusive base plate (aluminium foil), adhesive foam pad (flexible polyurethane) with occlusive base plate (aluminium foil disc) etc.
TRANSDERMAL PATCHES(16)
The first Transdermal patch was approved by the FDA in 1979. It was a patch for the treatment of motion sickness. In the mid-1980s, the pharmaceutical companies started the development of a nicotine patch to help smokers quit smoking, and within a few months at the end of 1991 and beginning of 1992 the FDA approved four nicotine patches. Transdermal Drug Delivery System has been a great field of interest in the recent time. Many drugs which can be injected directly into the blood stream via skin have been formulated. The main advantages of this system are that there is controlled release of the drug and the medication is painless. The drug is mainly delivered to the skin with the help of a Transdermal patch which adheres to the skin. A Transdermal Patch has several components like liners, adherents, drug reservoirs, drug release membrane etc. which play a vital role in the release of the drug via skin. Various types of patches along with various methods of applications have been discovered to delivery the drug from the transdermal patch. Because of its great advantages, it has become one of the highly research field among the various drug delivery system. Here, a general view over the transdermal patch has been discussed along with its advantages, disadvantages, methods of applying, care taken while applying, types and applications of transdermal patch and recent advances along with recent patents and market products.
DEVELOPMENT(17)
Before these patches go into the market, they have to be carefully studied. One way to study these patches are through the use of Franz Diffusion Cell systems. This system is used to study the effects of temperature on the permeated amount of a specific drug on a certain type of membrane, which in this case would be the membrane that is used in the patches. A Franz Diffusion Cell system is composed of a receptor and a donor cell. In many of these research studies the following procedure is used. The donor cell is set at a specific temperature (the temperature of the body), while the receptor cell is set at different one (temperature of the environment).Different runs are performed using different temperatures to study the impact of temperature on the release of a certain medicament through a certain type of membrane. Although different concentrations of the medicament are used in this study, they do not affect the amount permeated through the membrane (the process is constant). From Chemical kinetics it’s concluded that these studies are zero order, since the concentration plays no role in the permeated amount through the membrane. Some pharmaceuticals must be combined with substances, such as alcohol, within the patch to increase their ability to penetrate the skin in order to be used in a transdermal patch. Others can overwhelm the body if applied in only one place, and are often cut into sections and applied to different parts of the body to avoid this, such as nitroglycerin. Many molecules, however, such as insulin, are too large to pass through the skin.
ADVERSE EVENTS (18)
1-In 2005, the FDA announced that they are investigating reports of death and other serious adverse events related to narcotic overdose in patients using Duragesic, the fentanyl transdermal patch for pain control. The Duragesic product label was subsequently updated to add safety information in June 2005.
2- In 2009, the FDA announced a public health advisory warning of the risk of burns during MRI scans from transdermal drug patches with metallic backings. Patients should be advised to remove any medicated patch prior to an MRI scan and replace it with a new patch after the scan is complete.
REGULATORY ASPECTS(18)
A transdermal patch is classified by the U.S. Food and Drug Administration as a combination product, consisting of a medical device combined with a drug or biological product that the device is designed to deliver. Prior to sale in the United States, any transdermal patch product must apply for and receive approval from the Food and Drug Administration, demonstrating safety and efficacy for its intended.
DEFINITION
1-A transdermal patch or skin patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream.
Fig-2 Transdermal patch or Skin patch
The first commercially available prescription patch was approved by the U.S. Food and Drug Administration in December 1979, which administered scopolamine for motion sickness. (16, 17, 18)
DESIRABLE FEATURES FOR TRANSDERMAL PATCHES(19)
1. Composition relatively invariant in use.
2. System size reasonable.
3. Defined site for application.
4. Application technique highly reproducible.
5. Delivery is (typically) zero order.
6. Delivery is efficient.
THE MAIN COMPONENTS OF TRANSDERMAL PATCHES(13)
1- Liner - Protects the patch during storage. The liner is removed prior to use.
2- Drug - Drug solution in direct contact with release liner
3- Adhesive - Serves to adhere the components of the patch together along with adhering the patch to the skin
4- Membrane - Controls the release of the drug from the reservoir and multi-layer patches
5- Backing - Protects the patch from the outer environment
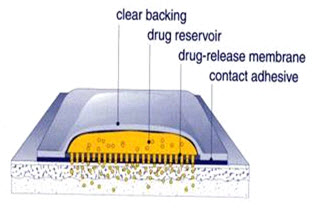
Fig-3 showing main components of transdermal patches
TYPES OF TRANSDERMAL PATCHES(15, 20, 21
Five Major Transdermal Systems:
1. Single-layer Drug-in-Adhesive
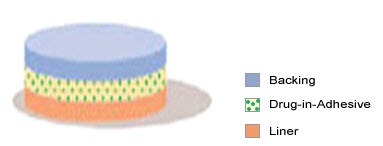
Fig-4 single layer drug-in-adhesive type
The Single-layer Drug-in-Adhesive system is characterized by the inclusion of the drug directly within the skin-contacting adhesive. In this transdermal system design, the adhesive not only serves to affix the system to the skin, but also serves as the formulation, containing the drug and all excipients under a single backing film. The rate of release of drug from this type of system is dependent on the diffusion across the skin.
The intrinsic rate of drug release from this type of drug delivery system is defined by
Cr
DQ/dT = ----------------
1/Pm + 1/Pa
where Cr is the drug concentration in the reservoir compartment and Pa and P m are the permeability coefficients of the adhesive layer and the rate controlling membrane , Pm is the sum of permeability coefficients simultaneous penetrations across the pores and the polymeric material. Pm and Pa, respectively, are defined as follow
Km/r. Dm
Pm = ____________
hm
Ka/m . Da
Pa = ___________
ha
where Km/r and Ka/m are the partition coefficients for the interfacial partitioning
of drug from the reservoir to the membrane and from the membrane to adhesive respectively; Dm and Da are the diffusion coefficients in the rate controlling membrane and adhesive layer, respectively; and hm and ha are the thicknesses of the rate controlling membrane and adhesive layer, respectively.
2. Multi-layer Drug-in-Adhesive
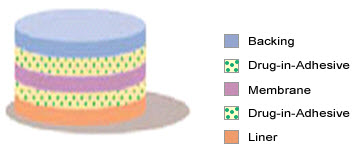
Fig-5 multi-layer drug-in-adhesive
The Multi-layer Drug-in-Adhesive is similar to the Single-layer Drug-in-Adhesive in that the drug is incorporated directly into the adhesive. However, the multi-layer encompasses either the addition of a membrane between two distinct drug-in-adhesive layers or the addition of multiple drug-in-adhesive layers under a single backing film.
The rate of drug release in this system is defined by:
Ka/r . Da
dQ/dt = ------------ Cr
ha
where Ka/r is the partition coefficient for the interfacial partitioning of the drug from the reservoir layer to adhesive layer.
3. Drug Reservoir-in-Adhesive
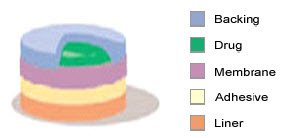
Fig-6 drug reservoir-in-adhesive type
The Reservoir Transdermal system design is characterized by the inclusion of a liquid compartment containing a drug solution or suspension separated from the release liner by a semi-permeable membrane and adhesive. The adhesive component of the product responsible for skin adhesion can either be incorporated as a continuous layer between the membrane and the release liner or in a concentric configuration around the membrane.
The rate of drug release from this drug reservoir gradient controlled system is given by:
Ka/r . Da
dQ/dt = --------------- A ( ha )
ha ( t )
In the above equation, the thickness of the adhesive layer for drug molecules to diffuse through increases with time ha (t). To compensate for this time dependent increase in the diffusional path due to the depletion of drug dose by release, the drug loading level is also increased with the thickness of diffusional path A (ha).
4. Drug Matrix-in-Adhesive
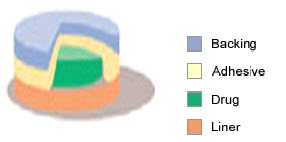
Fig-7 drug matrix-in-adhesive type
The Matrix system design is characterized by the inclusion of a semisolid matrix containing a drug solution or suspension which is in direct contact with the release liner. The component responsible for skin adhesion is incorporated in an overlay and forms a concentric configuration around the semisolid matrix.
The rate of drug release from this type of system is defined as:
dQ ACp Dp ½
--------- = ----------------
dt 2t
where A is the initial drug loading dose dispersed in the polymer matrix and Cp and DP are the solubility and diffusivity of the drug in the polymer respectively. Since, only the drug species dissolved in the polymer can release, CP in essentially equal to CR, where CR is the drug concentration in the reservoir compartment.
5. Vapour Patch
In this type of patch the adhesive layer not only serves to adhere the various layers together but also to release vapour. The vapour patches are new on the market and they release essential oils for up to 6 hours. The vapours patches release essential oils and are used in cases of decongestion mainly. Other vapour patches on the market are controller vapour patches that improve the quality of sleep. Vapour patches that reduce the quantity of cigarettes that one smokes in a month are also available on the market.
EVALUATION OF TRANSDERMAL PATCHES (22)
Evaluation of Pressure-Sensitive Adhesives Properties
The pressure-sensitive adhesives are evaluated for general adhesive properties as well as for dermal toxicity and human wear.
A- Adhesive Properties:
Pressure-sensitive adhesive can be evaluated on the basis of their three basic properties:
1. Peel Adhesion Properties
2. Tack Adhesion Properties
3. Shear-Strength properties
1. Peel Adhesion Properties:
The force required to remove an adhesive coating from a test substrate is referred to as peel adhesion. Molecular weight of adhesive polymer, the type and amount of additives, i.e. tackifiers and polymer composition are the variables that determine the peel adhesion properties. A single tape is applied to a stainless steel plate or a backing membrane of choice and then tape is pulled from the substrate at a 180 degree angle, and the force required for tape removed is measured. The force is expressed in ounces (or grams) per inch width of tape, with higher values indicating greater bond strength. If the pulled tape dose not leaves any residue on the plate, it indicates “adhesive failure,” If some residue is left behind, it suggests “cohesive failure”, which often signifies a lack of cohesive strength.
2. Tack Adhesion Properties:
Tack is the ability of a polymer to adhere to a substrate with little contact pressure. Molecular weight, composition of polymer and tackifying resins affect the tack properties of TDD systems.
There are four generally used tests for tack determination namely:
1- Thumb Tack Test
2- Rolling Ball Tack Test
3- Quick-stick(peel-tack) Test
4- Probe Tack Test
Thumb Tack Test:
In a qualities test applied for tack property determination of adhesive. In this test, the thumb is simply pressed on the adhesive and the relative tack property is detected.
Rolling Ball Tack Test:
This test measures the softness of a polymer that relates to tack. In this test, a stainless steel ball of 7/16 inches in diameter is released on an inclined track so that it rolls down and comes into contact with horizontal, upward-facing adhesive. The distance the ball travels along the adhesive provides the measurement of tack, which is usually expressed in inch. The less tacky the adhesive, farther the ball will travel.
Quick- stick (peel- tack) Test:
In this test, the tape is pulled away from the substrate at 90 degree Celsius at a speed of 12 inches/min. The forced required breaking the bond between adhesive and substrate is measured and recorded as tack value, which is expressed in ounces (or grams) per inch width. The higher value of force required indicates the higher degree of tack.
Probe Tack Test:
In this test probe tack tester is used. The tip of a clean probe with a defined surface roughness is brought into contact with adhesive, and when a bond is formed between probe and adhesive. The subsequent removal of the probe mechanically breaks it. The force required to pull the probe away from the adhesive at fixed rate is recorded as tack (expressed in grams).
3. Shear Strength Properties:
Shear strength is the measurement of the cohesive strength of an adhesive polymer. If transdermal device has adequate cohesive strength, it will not slip after application and will leave no residue upon removal. In this particular, adhesive-coated tape is applied onto a stainless steel plate. A specified weight is hung from the tape, to affect its pulling in a direction parallel to the plate. The longer the time taken for removal, greater is the shear- strength.
MECANISM OF ACTION OF TRANSDERMAL PATCH
The application of the transdermal patch and the flow of the active drug constituent from the patch to the circulatory system via skin occur through various methods.
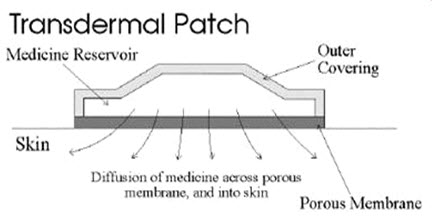
Fig-8 showing application of transdermal patch
1. Iontophoresis(23)
Iontophoresis passes a few milliamperes of current to a few square centimeters of skin through the electrode placed in contact with the formulation, which facilitates drug delivery across the barrier. Mainly used of pilocarpine delivery to induce sweating as part of cystic fibrosis diagnostic test. Iontophoretic delivery of lidocaine appears to be a promising approach for rapid onset of anesthesia.
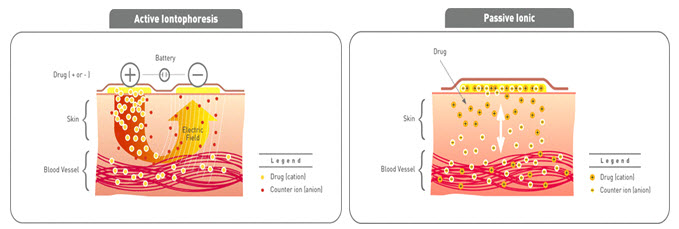
Fig-9 Iontophoresis
2. Electroporation(23, 24, 25, 26, 27)
Electroporation is a method of application of short, high-voltage electrical pulses to the skin. After electroporation, the permeability of the skin for diffusion of drugs is increased by 4 orders of magnitude. The electrical pulses are believed to form transient aqueous pores in the stratum corneum, through which drug transport occurs. It is safe and the electrical pulses can be administered painlessly using closely spaced electrodes to constrain the electric field within the nerve-free stratum corneum.
3. Application by ultrasound(23, 28)
Application of ultrasound, particularly low frequency ultrasound, has been shown to enhance transdermal transport of various drugs including macromolecules. It is also known as sonophoresis. Katz et al. reported on the use of low-frequency sonophoresis for topical delivery of EMLA cream.
Fig-10 Therapeutic Ultrasound Application
4. Use of microscopic projection(23)
Transdermal patches with microscopic projections called microneedles were used to facilitate transdermal drug transport. Needles ranging from approximately 10-100 µm in length are arranged in arrays. When pressed into the skin, the arrays make microscopic punctures that are large enough to deliver macromolecules, but small enough that the patient does not feel the penetration or pain. The drug is surface coated on the microneedles to aid in rapid absorption. They are used in development of cutaneous vaccines for tetanus and influenza. Various other methods are also used for the application of the transdermal patches like thermal portion, magnetophoresis, and photomechanical waves. However, these methods are in their early stage of development and required further detail studying.
FACTORS AFFECTING TRANSDERMAL BIOAVAILABILITY
Two major factors affect the bioavaibility of the drug via Transdermal routes:
A- Physiological factors
B- Formulation factors
Physiological factors include
1. Stratum corneum layer of the skin
2. Anatomic site of application on the body
3. Skin condition and disease
4. Age of the patient
5. Skin metabolism
6. Desquamation (peeling or flaking of the surface of the skin)
7. Skin irritation and sensitization
8. Race
Formulation factors include
1. Physical chemistry of transport
2. Vehicles and membrane used
3. Penetration enhancers used
4. Method of application
5. Device used
MARKETED PRODUCTS OF TRANSDERMAL PATCHES
MARKETED PRODUCTS OF TRANSDERMAL PATCHES
|
Brand Name |
Drug |
Manufacturer |
Indications |
||||
|
NicotinellR |
Nicotine |
Novartis |
Pharmacological smoking cessation |
||||
|
MatrifenR |
Fentanyl |
Nycomed |
Pain relief patch |
||||
|
Ortho EvraTM |
Norelgostromin/ Ethinyl Estradiol |
ORTHO-McNEIL |
Postmenstrual syndrome |
||||
|
NuPatch 100 |
Diclofenac diethylamine |
Zydus Cadila |
Anti Inflammatory |
||||
|
NeuproR |
Rigotine |
UCB and Schwarz Pharma |
early-stage idiopathic Parkinson’s disease |
||||
|
Alora |
Estradiol |
TheraTech/ Proctol and Gamble |
Postmenstrual syndrome |
||||
|
NicodermR |
Nicotine |
Alza/GlaxoSmithKline |
Smoking cessation |
||||
|
Estraderm |
Estradiol |
Alza/Norvatis |
Postmenstrual syndrome |
||||
|
Climara |
Estradiol |
3M Pharmaceuticals /Berlex Labs |
Postmenstrual syndrome |
||||
|
Androderm |
Testosterone |
TheraTech/ GlaxoSmithKline |
Hypogonadism in males |
||||
|
Nitrodisc |
Nitroglycerin |
Roberts Pharmaceuticals |
Angina pectoris |
||||
|
Transderm- ScopR |
Scopolamine |
Alza/Norvatis |
Motion sickness |
||||
|
Nuvelle TS |
Estrogen/ Progesterone |
Ethical Holdings/ Schering |
Hormone replacement therapy |
||||
|
Deponit |
Nitroglycerin |
Schwarz-Pharma |
Angina pectoris |
||||
|
Nitro-dur |
Nitroglycerin |
Key Pharmaceuticals |
Angina pectoris |
||||
|
Catapres TTSR |
Clonidine |
Alza/Boehinger Ingelheim |
Hypertension |
||||
|
FemPatch |
Estradiol |
Parke-Davis |
Postmenstrual syndrome |
||||
|
Minitran
Climaderm |
Nitroglycerin
Estradiol |
3M Pharmaceuticals
Ethical Holdings/ Wyeth-Ayerest |
Angina pectoris
Postmenstrual syndrome |
||||
|
DuragesicR |
Fentanyl |
Alza/Janssen Pharmaceutical |
Moderate/ severe pain |
||||
|
Estraderm |
Estradiol |
Alza/Norvatis |
Postmenstrual syndrome |
||||
|
Fematrix |
Estrogen |
Ethical Holdings/Solvay Healthcare Ltd. |
Postmenstrual syndrome |
||||
|
Transderm- NitroR |
Nitroglycerin |
Alza/Norvatis |
Angina pectoris |
||||
|
Testoderm TTSR |
Testosterone |
Alza |
Hypogonadism in males |
||||
|
OxytrolR |
oxybutynin |
Watson Pharma |
Overactive bladder |
||||
|
Prostep |
Nicotine |
Elan Corp./Lederle Labs |
Smoking cessation |
Table-1 Marketed product of Transdermal drugs
CONCLUSION
Transdermal drug delivery is hardly an old technology, and the technology no longer is just adhesive patches. Due to the recent advances in technology and the incorporation of the drug to the site of action without rupturing the skin membrane .Transdermal route is becoming the most widely accepted route of drug administration. It promises to eliminate needles for administration of a wide variety of drugs in the future. Transdermal ultrasound-mediated drug delivery has been studied as a method for needle-less, non-invasive drug administration. Potential obstacles include the stratum corneum, which is not sufficiently passively permeable to allow effective transfer of many medications into the bloodstream without active methods. A general review of the transdermal ultrasound drug delivery literature has shown that this technology offers promising potential for non-invasive drug administration. Included in this review are the reported acoustic parameters used for achieving delivery, along with the known intensities and exposure times. Ultrasound mechanisms are discussed as well as spatial field characteristics. Accurate and precise quantification of the acoustic field used in drug delivery experiments is essential to ensure safety versus efficacy and to avoid potentially harmful bioeffects.
REFERENCES
1. Chien, YW, Novel drug delivery systems, Drugs and the Pharmaceutical Sciences,Vol.50, Marcel Dekker, New York, NY;1992;797
2. Roberts MS, Targeted drug delivery to the skin and deeper tissues: role of physiology, solute structure and disease.Clin Exp Pharmacol Physiol 1997 Nov; 24(11):874-9.
3. Error! Hyperlink reference not valid.Transdermal Drug Delivery Systems:Introduction Pharmacokinetics, Clinical Efficacy, and Tolerance Development, In: Hadgraft, J., Guy, R.H., Eds., Transdermal Drug Delivery: Developmental Issues and Research Initiatives, Marcel Dekker, New York, 1989,135S.
4. Journal of Pharmacuetical Research published by Springer Netherlands, Vol.25, Issue No.5, Year 2008.
5. Journal of Pharmacuetical Research published by Springer Netherlands, Vol.25, Issue 11 Nov., Year 2008, Pg no.2697-2704.
6. Indian Journal of Pharmacuetical Sciences, Vol.69, Issue 4, Year 2007, Pg no. 535-539.
7. Asian Journal of Pharmacuetics Sciences published online 2007, 2(6): pg no. 249-259, ISSN 1818-0876.
8. Journal of Pharmacuetical Research published by Springer Netherlands, Vol.22, Issue April, Year 2005, Pg no.4.
9. European Journal of Pharmacuetical Science, Vol.18, Issue-Jan, Year 2003, Pg no.71-79.
10. European Journal of Pharmacuetics and Biopharmacuetics, Vol.54, Issue 2 sept., Year 2002, Pg no.161-164.
11. Indian Journal of Pharmacology, Issue 2000, Chapter-32, Pg no. 309-312.
12. International Journal of Pharmaceutics published by Elsevier Science, Vol.130, Issue 2 March, Year 1996, Pg no.169-177,
13. Yury Bayarski is the author of OriginalDrugs.com - website, offering patches and natural health product.
14. Banker, G. S and Rhodes, C. T Modern pharmaceutics, third edition, New York, Marcel Dekker, inc,. 1990.
15. Jain.N.K, Controlled and novel drug delivery, first edition, CBS publishers and distributors, New Delhi.1997.
16. Segal, Marian. "Patches, Pumps and Timed Release: New Ways to Deliver Drugs". Published by Marshall Cavendish, 2004, Pg: 2259
17. FDA ALERT (07/2005): Narcotic Overdose and Death". Food and Drug Administration.2005-07-15. http://www.fda.gov/cder/drug/InfoSheets/HCP/fentanylHCP.htm. Retrieved on 2007-02-24.
18. FDA Public Health Advisory: Risk of Burns during MRI Scans from Transdermal Drug Patches with Metallic Backings". http://www.fda.gov/cder/drug/advisory/transdermalpatch.htm. Retrieved on March 9, 2009
19. http://images.google.co.in/imgres?imgurl=http://www.chimei.org.tw/main/r...
20. Mathiowitz.Z.E, Chickering.D.E, Lehr.C.M, Bioadhesive drug delivery systems; fundamentals,novel approaches and development, Marcel Dekker, inc New York . Basel
21. 3M World Wide, 3M Drug delivery system, Transdermal patches, www.3Mworldwide.com
22. Vyas S.P. Theory and practice in Novel Drug Delivery System first edition Published by Satish kumar,2009, pg no.91-94
23. MedscapeTodayjournal Available http://www.medscape.com/viewarticle/530060_4
24. The Indian Anaesthetists'Forum-On-Line Journal (http://www.theiaforum.org/ ) April 2004 available http://www.theiaforum.org/april2004.htm
25. Electroporation From Wikipedia, the free encyclopedia available http://en.wikipedia.org/wiki/Electroporation
26. Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH (1982). "Gene transfer into mouse lyoma cells by electroporation in high electric fields". EMBO J. 1 (7): 841–5. PMID 6329708.
27. Sugar IP, Neumann E (1984). "Stochastic model for electric field-induced membrane pores. Electroporation". Biophys. Chem. 19 (3): 211–25. doi : 10.1016/0301-4622(84)87003-9. PMID 6722274.
28. Sarah Yang (2007 - 02-12). “New medical technique punches holes in cells, could treat tumors". Retrieved on 2007 - 12-13.
29. Jacoby, David B., Youngson, R. M., Marshall Cavendish Corporation; Encyclopedia Of Family Health, pharmacists and Pharmaceutical Scientists, Published by Taylor & Francis, 2001 Pg 216
30. Williams, Adrian; Transdermal and Topical Drug Delivery Published by Pharmaceutical Press, 2003; Pg: 14-18
31. Hillery, Anya M, Lloyd, Andrew W., Swarbrick, James; Drug Delivery and Targeting for Pharmacists and Pharmaceutical Scientists, Published by Taylor & Francis, 2001 Pg 216









