ABOUT AUTHOR:
Arya K.R.
Department of Pharmaceutics, Regional Institute of Medical Sciences and Research,
MG University, Kottayam
aryajithu@yahoo.com
ABSTRACT
Most ocular diseases are treated with topical eye drops. The poor bioavailability and therapeutic response exhibited by these conventional eye drops due to rapid precorneal elimination of the drug may be overcome by the use of insitu gelling systems that are instilled as drops into the eye and undergo a sol-to-gel transition in the cul-de-sac. The aim of the present investigation is to formulate and evaluate in situ ocular gelling system of Moxifloxacin hydrochloride and Ketorolac tromethamine using Gellan gum as gelling polymer and Hydroxypropyl methyl cellulose(HPMC K4M) as release retardant. The formulations were evaluated for clarity, pH measurement, gelling capacity, drug content estimation, rheological study, in vitro drug release and sterility testing. Results showed that all formulations were clear and showed pH within the range of 6.0-7.4. All formulation exhibited pseudo plastic rheology.Six different formulations with different concentration of Gellan gum(0.1%w/v,0.3%w/v,0.5%w/v) and HPMC K4M(0.1%w/v,0.2%w/v)were prepared which was found to have drug content of 99-100% for both drugs.Gellan gum 0.3%w/v with0.1%w/v HPMC K4M formulation having 87.10%of Moxifloxacin hydrochloride and 94.17% of Ketorolac tromethamine showing sustained drug release up to12 hours.
REFERENCE ID: PHARMATUTOR-ART-2041
Introduction
Conventional ophthalmic formulations like solution, suspension and ointment have many disadvantages which result into poor bioavailability of drug in the ocular cavity.1 Poor bioavailability of drugs from ocular dosage form is mainly due to the precorneal loss factors which include tear dynamics,non-productive absorption, transient residence time in the cul-de-sac and the impermeability of the corneal epithelial membrane2. Due to these physiological and anatomical constraints only a small fraction of the administered drug(effectively 1% or even less of the instilled dose) is ocularly absorbed.3
Inorder to overcome the problem of conventional ocular therapy,newer delivery system using in situ gelling polymers have been developed to prolong the precorneal residence time of a drug and improve ocular bioavailability. These system consist of polymers that exhibit sol to gel phase transition due to change in specific physico-chemical parameter (pH, temperature, electrolyte composition). Depending on the method employed to cause sol to gel phase transition on the eye surface the following three types of systems are recognized, pH triggered system Eg:Carbopol, Polyox, Cellulose acetate phthalate, temperature dependant system Eg:pluronics,methyl cellulose, ion activated system Eg:Gelrite, sodium alginate4.
The principal advantage of in situ gels is that they can be easily administered with accurate and reproducible dose compared to that of preformed gels and have an advantage that they can be easily instilled in liquid form and are capable of prolonging the residence time.5
Moxifloxacin hydrochloride is broad spectrum antibacterial drug,which acts by inhibiting two enzymes involved in bacterial DNA synthesis.They are DNA topoisomerases and gyrase, that are essential for bacterial DNA replication6. Ketorolac tromethamine is a non steroidalanti inflammatory drug which has demonstrated analgesic, anti-inflammatory, antipyretic activity, inhibiting prostaglandin biosynthesis7. This combination is indicated for the treatment of post operative ocular infection and inflammation after cataract surgery.
Gellan gum is an anionic exocellular polysaccharide produced by Pseudomonas elodea, having the characteristic property of cation induced gelation. Gel formation takes place because of complexation with polyvalent cations(Ca2+) in lacrimal fluid8. Hydroxy propyl methylcellulose(HPMC K4M)is an odourless,creamy white fibrous or granular powder. It is used as release retarding agent in controlled drug delivery system9.
The aim of this study is to prepare in situ ophthalmic hydrogel of Moxifloxacin hydrochloride and Ketorolac tromethamine using Gellan gum as gelling agent and HPMC K4M as release retardant to enhance ocular bioavailability and reduce dose frequency, thereby increasing patient compliance.
Materials and Methods
Materials
Moxifloxcin hydrochloride was purchased from Chethana pharmaceuticals, Perinthalmanna, Kerala and Ketorolac tromethamine was purchased from Divis pharmaceuticals, Hyderabad, HPMC K4M and Gellan gum from Chemdyes Corporation, Rajkot. All other chemicals were of analytical grade.
Instuments used were Double beam UV visible spectrometer (UV-1800,Shimadzu, Japan), Brookfield SynchroLectricvicometer (LVT 70947 Brookfield engineering Laboratories, USA).
Methods
Fabrication of in situ gel forming system of Moxifloxacin hydrochloride and Ketorolac tromethamine.
Polymer solution was prepared by dispersing gellen gum in minimum quantity of water and heated to 900C until it dissolves and then cooled. To this added, HPMC K4M with continuous stirring using magnetic stirrer. The drug solution of Moxifloxacin hydrochloride(0.5%w/v) and ketorolac tromethamine(0.5%w/v) in calculated quantity of distilled, deionized water added during the mixing step. Adjusted pH to 6.8 using 0.1N sodium hydroxide. The prepared in situ gels were filled in glass vials closed with rubber closures and sterilized by autoclaving at1210C for 20 minutes10.
Table 1: Formulation table of Moxifloxacin Hydrochloride and Ketorolac tromethamine insitu hydrogel
|
Ingredients(%w/v)
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Moxifloxacin hydrochloride
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Ketorolac tromethamine
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Gellan gum
|
0.1 |
0.1 |
0.3 |
0.3 |
0.5 |
0.5 |
|
HPMC K4M
|
0.1 |
0.2 |
0.1 |
0.2 |
0.1 |
0.2 |
|
NaOH(0.1N) |
Quantity sufficient |
|||||
|
Distilled water |
Quantity sufficient to 100ml |
|||||
Evaluation of the in situ gel forming system of Moxifloxacin hydrochloride and Ketorolac tromethamine.
Appearance
All developed formulations were evaluated for colour and clarity by visual examination against a black and white background.
pH
All formulations were evaluated for its pH by using a Digital pH meter(MKVI,Systronics).11
In vitro gelation studies and Viscosity
Gelling capacity was determined by placing a drop of the system in a vial containing 2 ml of simulated tear fluid freshly prepared and equilibrated at 37oC and visually assessing the gel formation and noting the time for gelation and the time taken for the gel formed to redissolve.The composition of simulated tear fluid was sodium chloride 0.670g,sodium bicarbonate 0.200g,calcium chloride dihydrate 0.008g and purified deionized water quantity sufficient to 100 ml.
The viscosity was measured using a Brookfield SynchroLectric viscometer(LVT 70947 model,spindle no 2) in the small volume adapter.The viscosity measured at 30 rpm was used for the purpose of comparative evaluation.
Rheological studies
Viscosity of instilled formulation is an important factor in determing residence time of drug in the eye.The selected formulations were poured into the small sample adaptor of the Brookfield SynchroLectric viscometer(LVT 70947 model,spindle no 2) and the angular velocity increased from 6,12,30,60 rpm.The hierarcy of the angular velocity was reversed.The average of the two readings was used to calculate the viscosity10.
Drug content estimation
The drug content was determined by diluting 1 ml of the formulation to 50ml with STF pH7.4. Aliquot of 5ml was withdrawn and further diluted to 50 ml with STF. Moxifloxacin hydrochloride concentration was then determined at 289 nm and ketorolac tromethamine at 323.5 nm by using UV-Vis spectrophotometer.
In vitro release studies
The in vitrorelease of Moxifloxacin Hydrochloride and ketorolac tromethamine from the formulations was studied through cellophane membrane using an open end cylinder.The dissolution medium used was simulated tear fluid freshly prepared (pH 7.4).Cellophane membrane, previously soaked overnight in the dissolution medium, was tied to one end of a specifically designed glass cylinder (open at both ends and of 5 cm diameter). A 1 ml volume of the formulation was accurately pipetted into this assembly.The cylinder was attached to the metallic driveshaft and suspended in 50 ml of dissolution medium maintained at 37± 1°C so that the membrane just touched the receptor medium surface.The dissolution medium was stirred at 50 rpm using magnetic stirrer.Aliquots, each of 1 ml volume, were withdrawn at hourly intervals and replaced by an equal volume of the receptor medium.The aliquots were diluted with the receptor medium and analyzed by UV-Vis spectrophotometer at 289 nm and 323.5 nm.
Date treatment of dissolution studies(Kinetic analysis)
Various models like zero order,first order,Higuchi models,Korsemeyer and Peppas and Hixson crowell were tested for explaining the kinetics of drug release based on the release rate data.12
Sterility testing
Direct inoculation method was used.2 ml of liquid from test container was removed with a sterile pipette or with a sterile syringe or a needle.The test liquid was aseptically transferred to fluid thioglycollate medium (20 ml) and soyabean-casein digest medium(20 ml) separately.The liquid was mixed with the media.The inoculated media were incubated for not less than 14 days at 300C to 350C in the case of fluid thioglycollate medium and 200C to 250C in case of soyabean-casein digest medium.13
Ocular irritancy studies
HET-CAM TEST(Hen’s Egg Test-Chorioallantonicmembrane )method
Firstly desi chicken eggs are selected.Eggs are placed flat on to the incubator trays maintained at 370Cand rotate for 8 days to prevent the attachment of the embryo to one side of the eggs.Temperature and humidity were checked at same time on each day.On 9th day,candled the eggs and discarded the non-viable eggs.Then replaced the egg with large end upwards in the incubator but donot rotate.Thus ensuring accessibility to the chorioallantoin membrane .On 10th day prepared the eggs for assaying.
Assay procedure.
Take the opened egg out of the incubator,pour off 0.9% sodium chloride solution at 370C.Carefully removed the egg membrane without injuring any underlying blood vessels using tapered forceps.Added 0.3% of the prepared formulation to the CAM.Observed the reactions on the CAM over a period of 5 minutes.Monitored the appearance of CAM forHaemorrage(bleeding) Vasularlysis(blood vessel disintegration) Coagulation(protein denaturation intra and extra vascular) with the help of magnifying glass.Recorded in seconds time for each reaction to occur and calculated irritation score(I.S)
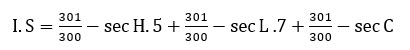
When determining the threshold the degree of severity of each reaction after treatment time has to be recorded according to the following scheme.14
0=no reaction 1=slight reaction 2=moderate reaction 3=severe reaction
Result and Discussion
Evaluation of the in situ gel forming system of Moxifloxacin hydrochloride and Ketorolac tromethamine.
According to the results listed in table 2, clarity of all prepared formulations was found to be satisfactory.The pH was within the acceptable range from 5.8 to 8.0) and hence would not cause any irritation upon administration.
In vitro gelation studies and viscosity.
The two main prerequisites of an insitu gelling system are viscosity andgelling capacity (speed and extend of gelation).The formulation should have an optimum viscosity that will allow easy instillation into the eye as a liquid(drops),which would undergo rapid sol-to-gel transition. To facilitate sustained release of drug to ocular tissue, the gel formed in situ should preserve its integrity without dissolving or eroding for a prolonged period of time.
Viscosity increased in proportion with viscofying agent but gelling agent had more effect on viscosity than viscofying agent.This may be attributed to the higher viscosity of Gellan gum.F3 showed immediate gelation and remained for extended period.Viscosity at 30rpm of this formulation was within the desirable range.So futher evaluation was carried on this formulation F3.
Table 2: Results of different evaluation parameters–Appearance,pH invitro gelation studies,viscosity
|
Formulation Code |
Clarity |
pH |
Gelling Capacity |
Viscosity at 30 rpm |
|
F1 |
clear |
6.9 |
+ |
20 |
|
F2 |
clear |
7.1 |
++ |
60 |
|
F3 |
clear |
7.3 |
+++ |
100 |
|
F4 |
clear |
7.4 |
+++ |
140 |
|
F5 |
clear |
7.2 |
+++ |
550 |
|
F6 |
Clear |
7.3 |
+++ |
690 |
Notice:+:Gels after few minutes and rapidly dissolves.
++:Immediately gels and remains for few hours.
+++:Immediately gels and remains for an extended time period.
Rheological studies
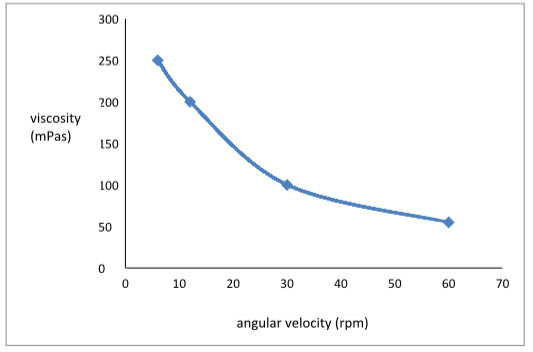
Formulation F3was shear thinning and an increase in shear stress was observed with increase in angular velocity(pseudoplastic rheology).The results are tabulated in Table 3 and graph 1 revealed that the viscosity decreases as the angular velocity increases.
Table 3:Rheological profile of insitu gelling system F3
|
Angular velocity(rpm) |
Viscosity(mPas) |
|
6 |
250 |
|
12 |
200 |
|
30 |
100 |
|
60 |
55 |
Graph 1: Rheological profile of insitu gelling system F3
Drug content estimation
The 99% of Moxifloxacin hydrochloride and 100.07% of ketorolac tromethamine was found in the prepared in situ hydrogel F3,indicating uniform distribution and maximum drug entrapment in the hydrogel.
In vitro release studies.
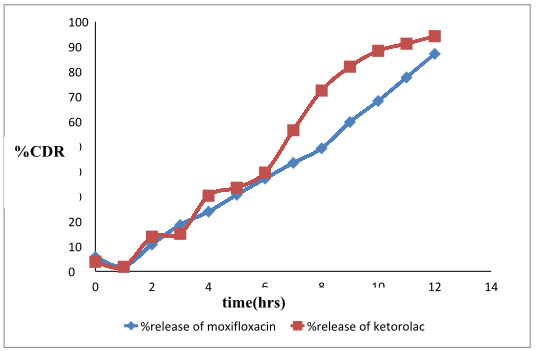
The results are tabulated in table 4 and a graph 2 is plotted. The results revealed that the Formulation F3 showed an initial burst release.The prolonged release in the later stage can be attributed to the slow diffusion of the drug through polymer matrix. The initial burst release of the drug can be explained by the fact that, thein situ gelling system is formulated in water and hence the polymer wascompletely hydrated. When they come in contact with STF, gelation occurs and a prehydrated matrix is formed in which hydration and water penetration no longer limit drug release, leading to an apparent diffusion-controlled release.
Table 4: Invitro release profile of in situ gelling formulation F3
|
Time(hours) |
% release of Moxifloxacin hydrochloride |
% release of Ketorolac tromethamine |
|
0 |
5.72 |
3.83 |
|
1 |
1.90 |
1.80 |
|
2 |
10.73 |
13.87 |
|
3 |
18.59 |
15.02 |
|
4 |
23.90 |
30.30 |
|
5 |
30.66 |
33.51 |
|
6 |
37.14 |
39.54 |
|
7 |
43.42 |
56.50 |
|
8 |
49.28 |
72.39 |
|
9 |
59.79 |
81.93 |
|
10 |
68.23 |
88.32 |
|
11 |
77.64 |
91.15 |
|
12 |
87.10 |
94.17 |
Graph 2: %cumulative release of Moxifloxacin hydrochloride and ketorolac tromethamine from in situ gelling formulation F3
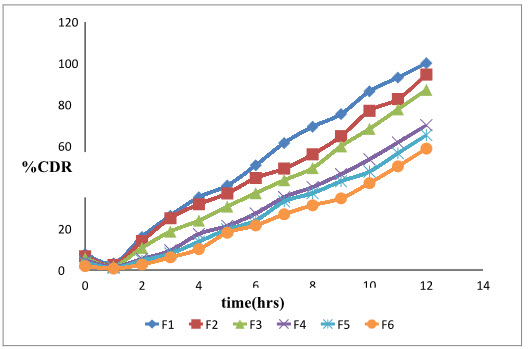
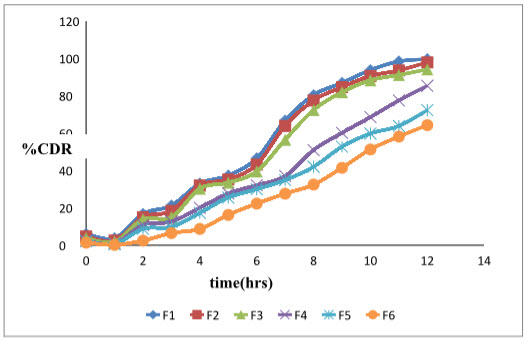
The graph 3 and graph 4 shows comparative release of Moxifloxacin hydrochloride and ketorolac tromethamine from formulations F1 –F6
Graph 3: % cumulative drug release profile of Moxifloxacin from formulations F1 –F6
Graph 4: % cumulative drug release profile of Ketorolac from formulations gum F1 –F6
Kinetics analysis
The results in table 5 revealed that F3 was fitted best to zero order drug release model
Table 5: Kinetic analysis of results of drug release of moxifloxacin hydrochloride and ketorolac tromethamine from formulation F3 by using 5 different models
|
Formulation Code |
Zero order r2 |
First order r2 |
Higuchi matrix r2 |
KorsmeyerPeppas |
Hixson-Crowellr2 |
Best fit model |
|
|
r2 |
n |
||||||
|
F3 Moxifloxcin |
0.984 |
0.878 |
0.849 |
0.963 |
1.387 |
0.922 |
Zero order |
|
Ketorolac |
0.972 |
0.901 |
0.856 |
0.953 |
1.457 |
0.941 |
Zero order |
Sterility testing
There was no appearance of turbidity and hence no evidence of microbial growth when the formulations were incubated for not less than 14 days at 300C to 350C and 200C to 250C in thioglycollate and soyabean-casein digest medium respectively.So the formulation F3 passes the test for sterility.
Ocular irritancy
In vivo eye irritation testing was carried out using eggs and as per HET-Cam test protocol. F3 was used for the test and was found to be non- irritating with no ocular damage with I.S value=0
Conclusion
Combination of Moxifloxacin and Ketorolac indicated for the treatment of post operative ocular infections and inflammation after cataract surgery was successfully formulated as in situ gel using gelrite as gelling polymer and hydroxypropyl methyl cellulose as release retardant.The formulated system provided sustained release of the drug for the more than 12 hours.The developed formulation is a viable alternative to conventional eye drop due to its ability to enhance bioavailability through its longer precorneal residence time and ability to sustain release of the drug.Also important is its ease of its administration and decreased frequency of administration resulting in better patient compliance.
References
1) Macha S.et. al.Ophthalmic drug delivery systems.Second edition Revised and expanded.Chapter 1.Overview of ocular drug delivery,1-3.
2) KaurI.P.et.al.Ocularpreparations:the formulation appoarch.Drug Dev. Ind.Pharm.2002,28,473-493.
3) Shell J.W. et.al.Ophthalmic drug delivery systems,Surv.ophthlmol.1984,29, 117.
4) Lekhrajvermaet.al.Development of phase change solutions for ophthalmic drug delivery based on ion activated and pH induced polymer,International Journal of Pharma professional’s reseach 2010,1(2),137-144.
5) Kavitha.Ket.al.Sustained ophthalmic delivery of Levofloxacin hemihydrate froman ion activated in situ gelling system, International Journal of pharm Tech Research, 2011,3(2),702-706.
6) BrennerG.M,Stevens C.W(2008),Pharmacology,3rd, Elsevier Pub,336.
7) Budavari.S91996),The Merck index,12th ed.5311,6292.
8) KulkarniM.C.et.al.Development of ophthalmic in situ gelling formulation of flubiprofen sodium using Gellan gum,Indian Drugs,2007,44(5)373-377.
9) Ainley wade and PaulJ.Weller, Handbook of Pharmaceutical Excipient’s, 2nd ed.229-331
10) Shastri D.H et.al.Design and Deveolpmentof Thermoreversible ophthalmic in situ hydrogel of Moxifloxacin hydrochloride,Current Drug Delivery,2010,7(3)238-243.
11) Rahul Nair et.al.Formulation and Evaluation of ion activated in situ gels of Vancomycin hydrochloride for ocular delivery,JITPs,2012,3(2),39-48.
12) VodithalaS.et.al.Formulation and Evaluation of ion activated ocular gel of Ketorolac tromethamine.International journal of current pharmaceutical research,2010,2(3),33-38.
13) B.P,2010,vol II,1224,1459.
14) LupkeN.P, Hen’s egg chorioallantonic membrane test for irritation potential, FoodChem Toxicol,1985,23,287.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









