ABOUT AUTHORS:
Raj Kishor
Avigna Clinical Research Institute
Bangalore
raryan859@gmail.com
ABSTRACT:
Achieving clinical trial research participant recruitment and retention is essential for conducting a successful trial. Adequate enrollment provides a base for projected participant retention, resulting in evaluative patient data. Obtaining final evaluative data is dependent on successful patient and principal investigator retention. Patients cannot be retained without an initial pool of enrolled volunteers.
REFERENCE ID: PHARMATUTOR-ART-2027
INTRODUCTION:
Motivate the patient in participating the study? It has to be learned first, that is the challenge in clinical research industry. Here neither the sponsor, CRO nor the site staffs are taking the time to understand the patient. We eat food we like because it tastes good. Has the Sponsor, the CRO or the Site has ever asked the patient how the protocol tastes? One strategy or one size does not fit for all.
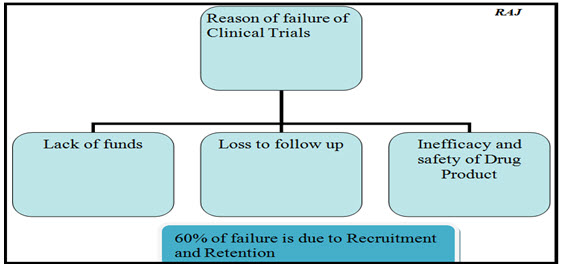
Differences in how effectively site enrolls or follow patients can skew the results and overly reflect the sites with the most data.
(NOTE): Despite more than two decades of focused attention and improvement efforts, patient recruitment and retention are among the largest challenges in clinical research enterprise is facing today and among the greatest contributors to delays in drug development.
RECRUITMENT: Is getting the attention of, and attracting potential research participants, i.e., “casting a net.”
RETENTION: Research participants remaining in the study for its duration, to provide valuable data and attain study objectives.It is also referred to as “Attrition” (participants leaving the study prematurely and/or being “lost to follow up”).
VARIOUS STEPS INVOLVED IN RECRUITMENT PROCESS
- Inclusion/Exclusion criteria
- Adequately explaining the study
- Recruiting an adequate and representative sample
- Obtaining true informed consent
- Maintaining ethical standards
- Retaining participants until study completion
- Minimizing cost-benefit ratio and Compensation
THE VARIOUS METHODS OF PATIENT RECRUITMENT
- Advertisements
- Honest Broker System
- Recruitment letters and/or scripts
- Research registry
- “Dear Doctor” Letters to encourage clinician referrals
- General public interest news items on diseases or conditions -not study specific
- Recruitment advertising that is intended to be seen or heard by prospective subjects to solicit their participation in a study
- Newspapers, local papers, publications for parents, etc.
- Television and radio
- Posters, flyers: post around campus, in public spaces with permission if necessary.
- Random digit Dialing (RDD) used in surveys and polling
- Pop-Ups, banner Ads with required IRB approval
What after recruitment?
Once participants are recruited to participate in the trial, retaining them becomes a key to success. All of the factors identified as important for recruitment are important for retention as well. A critical factor in retention is delivery on promises made during recruitment (e.g., that the burden of participation is low). Many factors need to be considered in developing a retention plan, including how long the patient is likely to return to the enrolling site. Maintaining ethical incentives for patient participation (ranging from newsletters to payments) is also important.The resources available for patient retention efforts should also be clear. Follow up rates can often be improved with more efforts; such as more attempts to contact the patient,but these efforts add cost.
STRATEGIES TO FACILITATE RETENTION:
- Inquire the patient about barriers to participation “Why…and is there anything we can do?”
- Flexible visit schedules like early morning, evenings and weekends
- Access visit windows
- Atmosphere of visit length of time spent in clinic
- Diversions–magazines, DVDs and refreshments
- Study staff and clinic staff interaction
- Develop tools for your team and make it as easy as possible to conduct the study procedures especially for clinical staff
- Communication with participants: honest and frequent education enables an informed decision
- Develop trust with the patients
- Reminder phone calls and e-mails
- Appreciation cards like –birthday cards, study anniversary cards and study tokens
- Thank you letters at study close and share results if allowed
PITFALLS IN RECRUITMENT AND RETENTION:
Pitfalls abound in recruitment and retention.The most important of these pitfalls is the risk of selection bias.Targeting hospital or academic-based physicians and excluding community-based physicians.This is tempting because the former are often more accessible and is frequently more open to involvement in, and more experienced in, research projects.Another major pitfall is confusing terminologies.Materials that are translated into other languages must undergo strict quality assurance measures to ensure that terms are translated properly i.e. as it is in simple terms.
BARRIERS OF RECRUITMENT AND RETENTION
Barriers are classified in four broad groups:
· Subject-related barriers
· Investigator-related barriers
· Protocol-related barriers and
· Other barriers.
Subject-related Barriers: Long waiting times associated with clinical visits and inconvenient scheduling of appointments is the main in this type of barrier. Subjects who have unrealistic expectations of the clinical trial may be reluctant to complete the study protocol.This may result in reduced retention, with subjects dropping out prior to trial completion.
Investigator-related Barriers: This may be divided into logistical factors and personal factors. Investigator’s underestimation of the workload involved in a clinical trial is the main cause here. This could reflect a poor choice of investigator to undertake the study, and could also imply poor communication between the sponsor company and the investigator as to the amount of work actually involved in the trial.A lack of time and resources to undertake the study may cause investigators to lose their motivation to recruit the patients which results in failure of the study.
Protocol-related Barriers: Lack of full support and keenness for the design or aims of the study protocol on behalf of the investigator are the main causes here.A protocol design whose eligibility criteria is rigid that do not qualify for entry of the potential study subjects and protocols that are too difficult for investigators to follow due to overly complex study designs are one of the major protocol-related barriers.
Other Barriers: The main other barrier are the negative influence of the media, ethical approval process that delay subject recruitment and trial commencement and the poor choice of study site by the sponsor.The importance of involving key opinion leaders in clinical trials has to be mentioned as a strategy to help endorse products.
According to a recent Center Watch report, dropout rates of 15-40% in clinical trials are not uncommon, and 86% of all U.S. clinical studies fail to recruit the required number of subjects on time. Participant recruitment to clinical trials has been called “the most difficult and challenging aspect of clinical trials,” and the blemishes in recruitment is identified as one of the main reasons for the failure of clinical studies.
CURRENT CHALLENGES IN CLINICAL TRIAL PATIENT RECRUITMENT AND ENROLLMENT
This can be measured from three perspectives namely:
- Sponsor perspective
- Principal Investigator perspective
- Clinical research volunteer perspective.
Sponsor Perspective: A research participant enrollment goal is established far in advance of trial initiation.The target number that is documented in the trial protocol for the number of desired participants is N and the number is small.Those who design the study plan must specify a larger number “N” in the protocol than the number of participants expected to be evaluated at study closure.
Principal Investigator Perspective: The Principal Investigator is the link between the patient and the sponsoring pharmaceutical company. Therefore a prime goal of the sponsor is to successfully recruit qualified Investigator. Commonlydrug companies and contractors offer large payments to doctors, nurses and other medical staff to recruit patients,some getting finder’s fees for merely referring patients to the study. This activity is strongly frowned upon in academic institutions and absolutely discouraged. In some rare instances the referring physician (usually a resident) will be given a ‘low-cost’ gift certificate to the medical bookstore.
Clinical Research Volunteer Perspective: Common barriers that potential volunteers consider in regards to clinical trial participation are lack of awareness of clinical trials, lack of access to trials,distrust or suspicions of research,practical or personal obstacles,Insurance or cost problems, Unwillingness to go against personal physician’s wish,long-standing fear, apprehension and skepticism. People from various cultural or ethnic backgrounds hold different values and beliefs that may be different.
RECENT ADVANCES: According to following prospects
- Professional recruitment providers
- Increased use of market research
- Informatics
- Centralized recruiting
- Development of Metrics
Professional recruitment providers: These are the companies that can structure media campaigns, designs and place advertisements and follow up the strategy as the study stretches out.
Increased use of market research: Market research can sort out populations based on demography, geography, psychology and behavior.With the help of market research, the sponsor can gain access to media behavior of their flock of patients and therefore focused media campaigns can be developed for the targeted population.
Informatics:It is a database-driven approach in finding potential subjects.It provides de-identified data obtained from pharmacies, physicians, hospitals and insurance companies etc. Once the population is defined, they would target the population with direct mail campaigns.
Centralized recruiting: It means that there is one centralized location for all information regarding potential subjects and it facilitates accurate tracking and analysis of recruitment services.
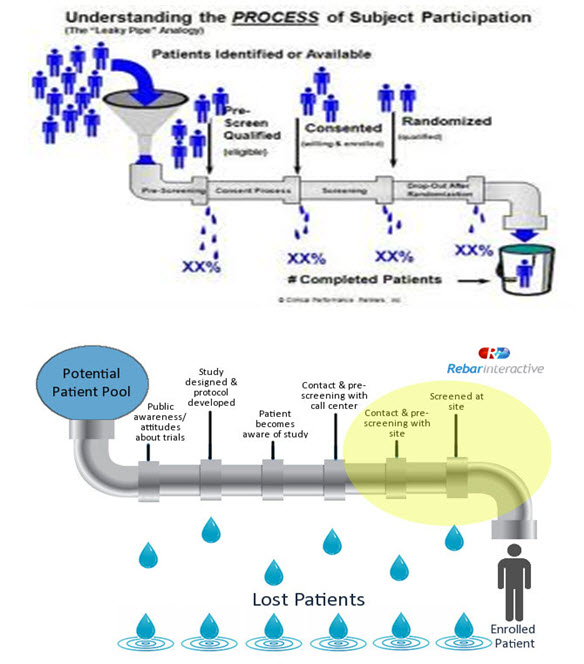
Development of Metrics:“The Leaky-pipe Analysis”
The metrics evaluate the effectiveness of a recruitment program.Through continuous measurement of methods of patient recruitment,meaningful metrics will take shape.The Leaky-pipe analysis is a visual aid.Once the leaky areas of the pipe are identified, then it is needed to be repaired.This not only helps in tracking the recruitment process but also helps to repair when damages are done.
CONCLUSION:
At present time, as the number of trials is increasing, the number of patients required is also increasing.As the traditional recruitment and retention methods have failed to meet the goal, the recruitment of patients has become partnering process between the Sponsor, Investigator and the CRO.Exploring the difficulties of recruitment and retention and carefully planned design,implementation,and follow-through of sound recruitment and enrollment strSategies contribute to the efficiency and success of clinical research trials from initiation to study close-out.
REFERENCES:
1. Patient Recruitment In Clinical Trials. Editorial Review. amazon.com/exec/obidos/tg/ stores/detail/-/books/0881679313/ reviews/independentrevie/103-0887158-4826253. Viewed 06 June 03.
2. Schilsky, RL. Conversations in Care - Chapter 7, Meeting the Challenges of Clinical Trial Enrollment. Web-book. conversations incaes.com/ web_book/chapter07.html. Viewed 16 April 2003. Quoted from Langley C, Gray S, Selley S, Bowie C, PrinceC. Clinicians’ attitudes to randomized trials in cancer care: a qualitative study. J Health Serv Res Policy. 2000; 5(3) 164-169.
3. centerwatch.com/professional/ prv295.html.
4. appliedclinicaltrialsonline.com/appliedclinicaltrials/article/articleDetail.jsp?id=89608
5. socra.org/pdf/200402_Current_Challenges_Recruitment_Enrollment.pdf6. Basic principles of clinical research and methodology – Dr SK Gupta
7. L.C. Lovato, K. Hill, S. Hertert, B.D. Hunningshake, J.L. Probstfield, “Recruitment for Controlled Clinical Trials: Literature Summary and Annotated Bibliography,” Controlled Clinical Trials, 18, 328–357 (1997).
8. B. Spilker and J.A. Cramer, Patient Recruitment in Clinical Trials (Raven Press, New York, 1992).
9. D.L. Anderson, A Guide to Patient Recruitment—Today’s Best Practices and Proven Strategies (CenterWatch Inc., Boston, MA, 2001).
10. R.A. Nathan, “How Important is Patient Recruitment in Performing Clinical Trials?” Journal of Asthma, 36, 213–216 (1999).
11. M.E. Mattson, J.D. Curb, R. McArdle, AMIS & BHAT Research Groups, “Participation in a Clinical Trial: The Patients’ Point of View,”Controlled Clinical Trials, 6, 156–167 (1999).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









