About Authors:
Mr. Salahuddin Mohammed
Associate Professor of Pharmacology, College of Health Sciences,
Department of Pharmacy, Mizan Tepi University,
Mizan Teferi, Ethiopia.
salahuddin_pharma48@yahoo.com
Introduction
Secondary messenger system is a part of cellular signaling process in which proteins of different kind are activated through generation of diffusible signaling molecules. The activated proteins then participate in a cellular response.
Second messengers are produced catalytically in response to the extracellular signals (primary messengers) and amplify their response, thus second messengers are a part of signal transduction cascades.
REFERENCE ID: PHARMATUTOR-ART-2031
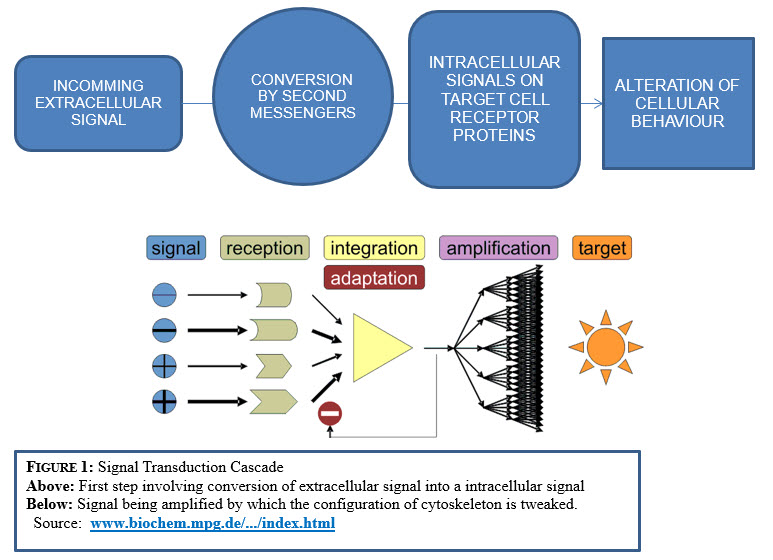
In the transduction process, extracellular signal is passed from one intracellular molecule to another through the secondary messengers until al cellular behavior alters and the cytoskeleton is tweaked into new configuration.
G-protein Linked Receptor activation
G-protein linked receptors activate a class of membrane bound proteins which then migrates in the plane of plasma membrane initiating the cascade effects. This results in enzymatic alteration and generates host of additional signals, called as second messengers.
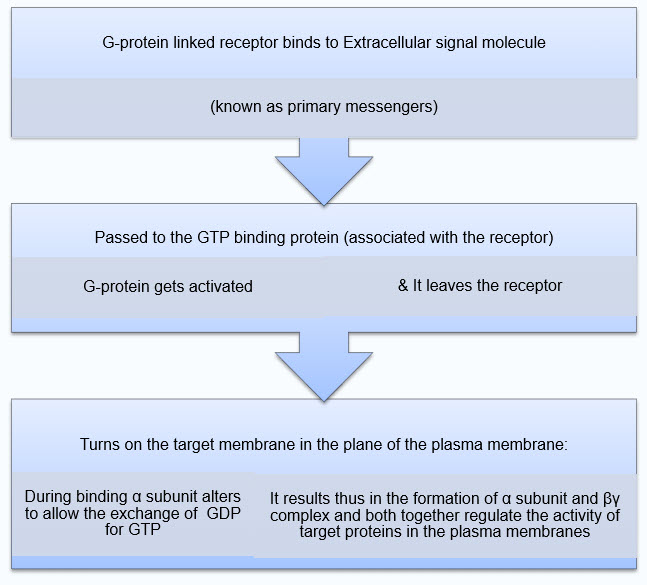
Figure 2: G-Protein receptor activation
· When the G-protein is activated it dissociates in to two signaling proteins.
· After the regulation of the target protein is completed, the G-protein α subunit switches off by hydrolyzing the bound GTP into GDP by GTPase. Then the reassociation of α and βγ complex takes place making the G-protein ready to couple with other receptor.
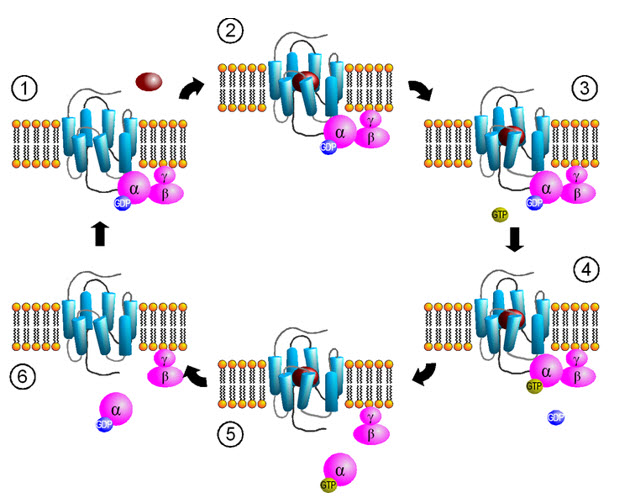
Figure 3: Activation cycle of G-proteins by G-protein-coupled receptors
Source by Sven Jähnichen
REQUIREMENTS FOR A COMPOUND TO ACT AS A SECOND MESSENGER
- During hormone-receptor binding, concentration of second messenger should increase in order to exert the biological effect.
- When the hormone is removed, the concentration of second messenger and the biological response shown by the hormone should decrease in concentration and activity.
type of intracellular signaling molecules
They are produced depending upon the interaction of the G-proteins with target enzymes as given below:
|
S.No |
TARGET ENZYME |
INTRACELLULAR SIGNALING MOLECULE PRODUCED |
|
1. |
Adenylyl cyclase |
Cyclic AMP |
|
2. |
Phospholipase C |
Inositol triphosphate |
|
3. |
Phospholipase C |
Diacylglycerol |
|
4. |
Guanylyl cyclase |
Cyclic GMP |
Table 1: Types of Intracellular signalling molecules
I. CYCLIC AMP AS A SECONDARY MESSENGER
Cyclic-3’,5’-adenosine monophosphate is second messenger in intracellular signaling cascade. Wide variety of exogenous stimuli, such as hormones , neurotransmitters, physical and chemical signals control the intracellular level of cyclic nucleotides by regulating the enzyme systems directly or indirectly. These physiological effects are exerted through binding to a number of proteins which includes cyclic nucleotide dependant protein kinases, cyclic nucleotide gated channels and cyclic nucleotide-regulated phosphodiesterases.The phosphorylated state of various proteins and cyclic nucleotides are controlled by extracellular signals.
The regulatory mechanism of adenylyl cyclase is a typical G-protein dependant signal transduction.
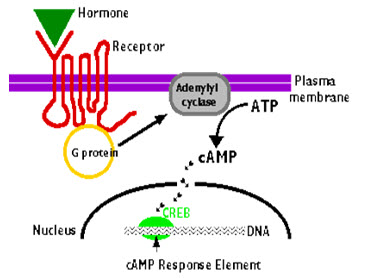
Figure 4: Cyclic AMP activation
Source: universe-review.ca/F11-monocell.htm
Regulation by G-protein subunits
G-protein consists of two functional subunits, Gs (stimulatory protein) and Gi (inhibitory protein). The GTP bound Gsα associates with adenylyl cyclase and stimulates the rate of cAMP synthesis and in contrast GTP bound Giα inhibits the catalytic activity.
The regulation of different adenylyl cyclases types are given below:
|
REGULATOR |
MODE OF REGULATION |
ADENYLYL CYCLASE TYPES |
|
Gsα |
STIMULATION |
1-6 |
|
Giα |
INHIBITION |
1-6 |
|
Gβγ |
STIMULATION |
2,4 |
|
FORSKOLIN |
INHIBITION |
1-6 |
|
CALMODULIN |
STIMULATION |
1,3,8 |
|
CALCIUM |
INHIBITION |
5,6 |
|
PROTEIN KINASE C |
STIMULATION |
2,5 |
|
PROTEIN KINASE A |
INHIBITION |
6 |
Table 2: Regulation of cyclic AMP by G Proteins
Regulation by calcium
Recent studies showed that some adenylyl cyclases are regulated by calcium/calmodulin. Type 1,3,8 enzymes are stimulated by calcium.Types 4 and 2 are not sensitive to calcium. Types 5 and 6 appear to be inhibited by low concentrations of free calcium.
Regulation by phosphorylation
Adenylyl cyclase is a direct target for phosphorylation by protein kinaseC, It alters the responsiveness to G-protein regulation.Adenylyl cyclase isoforms are differentially regulated by protein kinase A dependent phosphorylation.
The regulation of adenylyl cyclases by protein kinases is also connected to the regulatory mechanism of protein kinases including various growth factors and other activation pathways. Thus, adenylyl cyclases system is involved in many number of signal transduction systems.
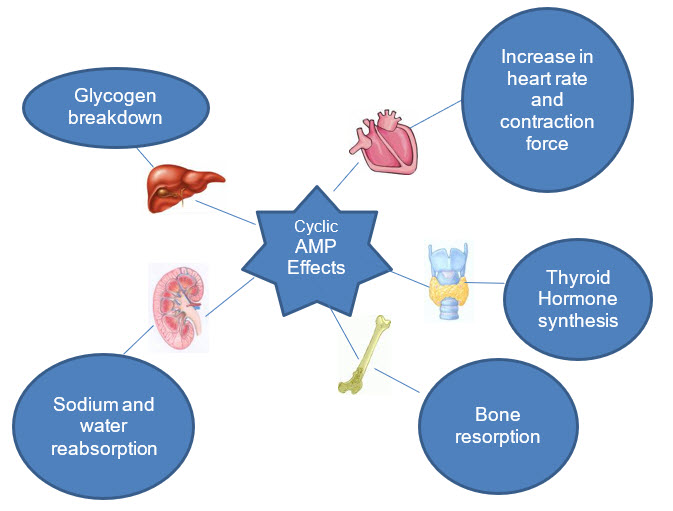
Figure 5: Effects of Cyclic AMP as a secondary messenger
Some effects of protein phosphorylation by c-AMP dependent protein kinase are listed in the table below:
|
ENZYME |
EFFECT |
|
Phosphorylase kinase |
Phosphorylation of phosphorylase and glycogen breakdown |
|
Glycogen synthetase |
Inhibition of glycogen synthesis |
|
Hormone sensitive triglyceride lipase |
Stimulation of triglyceride breakdown |
|
Smooth chain myosin light chain kinase |
Inhibition of contraction |
|
Phospholamban |
Increased calcium uptake into sarcoplasmic reticulum of heart muscle |
Table 3: Effects of protein phosphorylation by cyclic AMP dependent protein kinase.
II. LIPID DERIVED SECONDARY MESSENGERS: IP3 AND DAG
In this signaling cascade system, the extracellular signals activate phospholipase C instead of adenylyl cyclase through the G-protein receptors which results in the Inositol-phospholipid pathway which takes place as follows:
1. Activated phospholipase C cleaves PIP2 into DAG and IP3
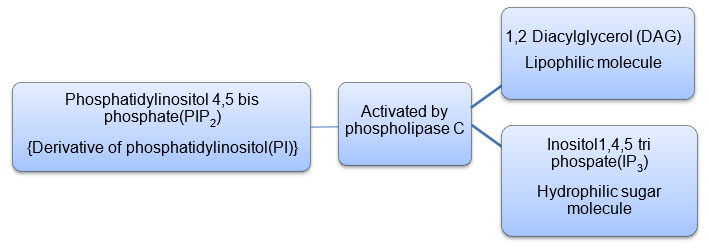
Figure 6: Formation of IP3 and DAG from PIP2
2. IP3 being a hydrophilic sugar molecule diffuses into the cytosol and attaches to the endoplasmic reticulum and opens the calcium channels embedded on the endoplasmic reticulum to cause a sharp increase in the cytosolic concentration of free calcium.
3. DAG together with the released calcium activates protein kinase C(PKC) causing its traslocation from the cytosol to the plasma membrane.
4. PKC phosphorylates set of intracellular proteins to exert their effects.
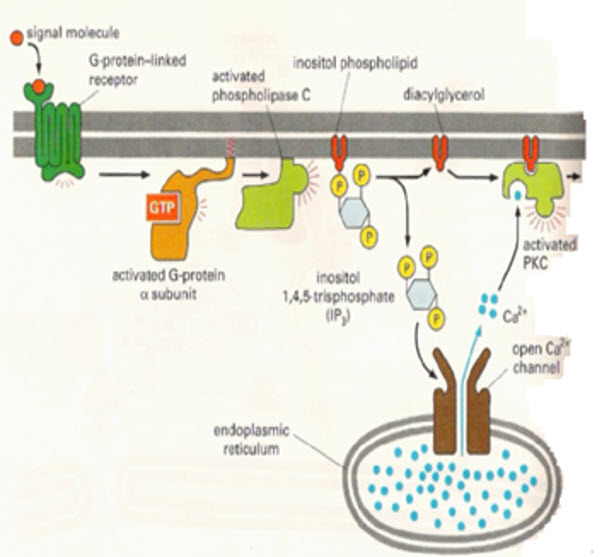
Figure 7: Activation of IP3 as a secondary messenger
SOME FIRST MESSENGERS WHICH ACTIVATE PI BREAKDOWN
|
MESSENGER |
TARGET CELLS |
PHYSIOLOGICAL EFFECTS |
|
Adrenaline, ATP Vasopressin |
LIVER(α1, P2,V1 receptors respectively) |
Glycogen breakdown |
|
Acetylcholine |
Exocrine pancreas |
Secretion |
|
Acetylcholine |
Smooth muscle |
Contraction |
|
PDGF |
Platelets |
Cell proliferation |
|
Thrombin |
Fibroblasts |
Aggregation |
Table 4: First messengers which activate PI breakdown.
EFFECTS OF CALCIUM RELEASE IN THE BODY
Calcium plays important role in embryonic development, acts on skeletal muscle and causes contraction. It also acts on nerve cells(secretary cells) and triggers secretion.
Indirectly calcium acts through transducer proteins where calcium binds to calmodulin and this complex activates the target proteins.
III. c-GMP SiGNALLING: NITRIC OXIDE and ATRIAL NATURETIC PEPTIDES
Several hormones like Insulin, Oxytocin elevate cGMP levels. Effects shown by cGMP are opposite to that shown by cAMP and increase in cGMP is often accompanied by decrease levels of cGMP.
Nitric Oxide and cGMP sinalling:
Ca -calmodulin complex results in activation of Nitric oxide synthase, resulting in release of nitric oxide (NO). Effect of NO on the smooth muscle is mediated by cGMP as follows:
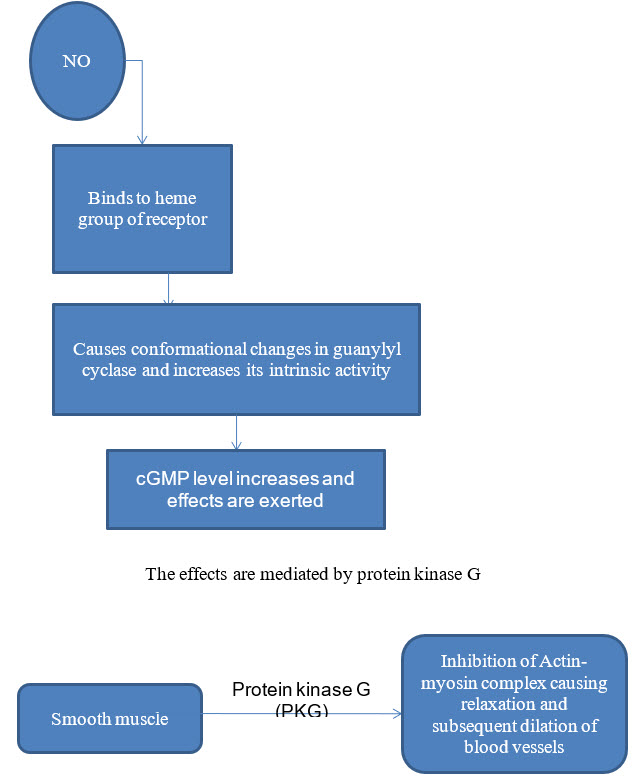
Figure 8: Effect of NO on the smooth muscle mediated by cGMP
Atrial Naturetic Factor(ANF) and other peptides:
Atrial naturetic factor is released from granules of cardiac atrial tissue and uses cGMP as a second messenger. When cGMP content increases, it activates membrane bound guanylate cyclase and inturn cGMP dependent protein kinase, which mediates activation of ANF by changing the shapes of both regulatory and catalytic site subunits.The stimulation of it subsequently causes relaxation of smooth muscle.
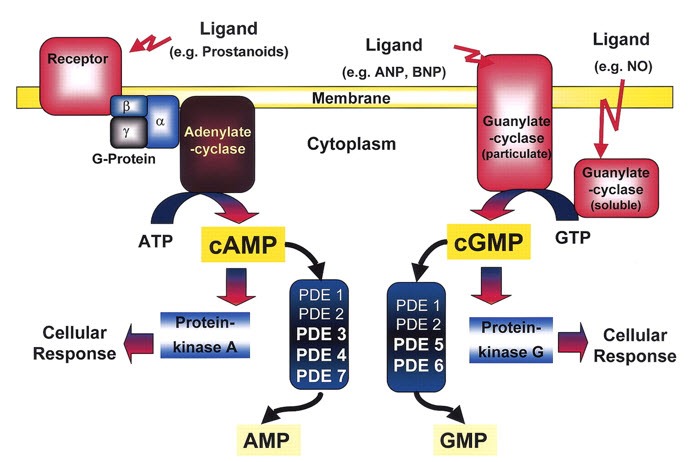
Figure 9: Cyclic GMP as a secondary messenger
REFERENCES
Internet links
1. biochem.mpg.de/.../index.html
2.universe-review.ca/F11-monocell.htm
3. biochem.mpg.de/.../index.html
Books
1.Ganong, W.F. Review of Medical Physiology (16th ed.). Appleton & Lange, 1993.
2.Chapter 1, pp. 30-40.
3.D.G. Hardie (1991). Biochemical Messengers: Hormones Neurotransmitters and Growth factors; 1st Edition; London: Chapman and Hall.
4.Gerald Karp(2001).Cell and Molecular Biology: Concepts and Experiments;3rd Edition; New York: John Wiley and Sons.
Journals
1.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439: 599–603, 2006.
2.Ahloulay M, Dechaux M, Hassler C, Bouby N, Bankir L. Cyclic AMP is a hepatorenal link influencing natriuresis and contributing to glucagon-induced hyperfiltration in rats. J Clin Invest 98: 2251–2258, 1996.
3.Ahlstrom M, Lamberg-Allardt C. Regulation of adenosine 3',5'-cyclic monophosphate (cAMP) accumulation in UMR-106 osteoblast-like cells: role of cAMP-phosphodiesterase and cAMP efflux. Biochem Pharmacol 58: 1335–1340, 1999.
4.Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5'-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology 147: 3435–3445, 2006.
5.Bankir L, Ahloulay M, Devreotes PN, Parent CA. Extracellular cAMP inhibits proximal reabsorption: are plasma membrane cAMP receptors involved? Am J Physiol Renal Physiol 282: F376–F392, 2002.
6.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000.
7.Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 8: 169–184, 2007.
8.Brown EM, Carroll RJ, Aurbach GD. Dopaminergic stimulation of cyclic AMP accumulation and parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci USA 74: 4210–4213, 1977.
9.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993.
10.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001.
11.Brundege JM, Diao L, Proctor WR, Dunwiddie TV. The role of cyclic AMP as a precursor of extracellular adenosine in the rat hippocampus. Neuropharmacology 36: 1201–1210, 1997.
12.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA 99: 1115–1122, 2002.
13.Caroppo R, Gerbino A, Debellis L, Kifor O, Soybel DI, Brown EM, Hofer AM, Curci S. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia. EMBO J 20: 6316–6326, 2001.
14.Caroppo R, Gerbino A, Fistetto G, Colella M, Debellis L, Hofer AM, Curci S. Extracellular calcium acts as a "third messenger" to regulate enzyme and alkaline secretion. J Cell Biol 166: 111–119, 2004.
15.Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol 291: G753–G761, 2006.
16.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–511, 2007.
17.Daniel EE, El-Yazbi A, Cho WJ. Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med 10: 529–544, 2006.
18.Davoren PR, Sutherland EW. The effect of L-epinephrine and other agents on the synthesis and release of adenosine 3',5'-phosphate by whole pigeon erythrocytes. J Biol Chem 238: 3009–3015, 1963.
19.De Luisi A, Hofer AM. Evidence that Ca2+ cycling by the plasma membrane Ca2+-ATPase increases the ‘excitability’ of the extracellular Ca2+-sensing receptor. J Cell Sci 116: 1527–1538, 2003.
20.Detrick MS, Kreisberg R, Koontz JW, Moore RN. Nanomolar cyclic adenosine and guanosine monophosphates stimulate macrophage colony-stimulating factor responsiveness by murine transitional progenitors. J Leukoc Biol 52: 249–254, 1992.
21.Do T, Sun Q, Beuve A, Kuzhikandathil EV. Extracellular cAMP inhibits D(1) dopamine receptor expression in CAD catecholaminergic cells via A(2a) adenosine receptors. J Neurochem 101: 619–631, 2007.
22.Elalamy I, Said FA, Singer M, Couetil JP, Hatmi M. Inhibition by extracellular cAMP of phorbol 12-myristate 13-acetate-induced prostaglandin H synthase-2 expression in human pulmonary microvascular endothelial cells. Involvement of an ecto-protein kinase A activity. J Biol Chem 275: 13662–13667, 2000.
23.Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 8: 1335–1338, 1998.
24.Gerbino A, Fistetto G, Colella M, Hofer AM, Debellis L, Caroppo R, Curci S. Real time measurements of water flow in amphibian gastric glands: modulation via the extracellular Ca2+-sensing receptor. J Biol Chem 282: 13477–13486, 2007.
25.Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca2+ and G(alpha)i via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations. J Cell Biol 171: 303–312, 2005.
26.Gherghiceanu M, Popescu LM. Caveolar nanospaces in smooth muscle cells. J Cell Mol Med 10: 519–528, 2006.
27.Godinho RO, Costa VL Jr. Regulation of intracellular cyclic AMP in skeletal muscle cells involves the efflux of cyclic nucleotide to the extracellular compartment. Br J Pharmacol 138: 995–1003, 2003.
28.Golstein PE, Daifi A, Crutzen R, Boom A, Van Driessche W, Beauwens R. Hypotonic cell swelling stimulates permeability to cAMP in a rat colonic cell line. Pflugers Arch 447: 845–854, 2004.
29.Gray E, Muller D, Squires PE, Asare-Anane H, Huang GC, Amiel S, Persaud SJ, Jones PM. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J Endocrinol 190: 703–710, 2006.
30.Hebert SC. Calcium and salinity sensing by the thick ascending limb: a journey from mammals to fish and back again. Kidney Int Suppl 91: S28–S33, 2004.
31.Hendy GN, Tomlinson S, O’Riordan JL. Impaired responsiveness to the effect of glucagon on plasma adenosine 3':5'-cyclic monophosphate in normal man. Eur J Clin Invest 7: 155–160, 1977.
32.Hofer AM. Another dimension to calcium signaling: a look at extracellular calcium. J Cell Sci 118: 855–862, 2005.
33.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538, 2003.
34.Hofer AM, Curci S, Doble MA, Brown EM, Soybel DI. Intercellular communication mediated by the extracellular calcium-sensing receptor. Nat Cell Biol 2: 392–398, 2000.
35.Hofer AM, Gerbino A, Caroppo R, Curci S. The extracellular calcium-sensing receptor and cell-cell signaling in epithelia. Cell Calcium 35: 297–306, 2004.
36.Jackson EK, Mi Z, Dubey RK. The extracellular cAMP-adenosine pathway significantly contributes to the in vivo production of adenosine. J Pharmacol Exp Ther 320: 117–123, 2007.
37.Jackson EK, Mi Z, Zacharia LC, Tofovic SP, Dubey RK. The pancreatoheptorenal cAMP-adenosine mechanism. J Pharmacol Exp Ther 321: 799–809, 2007.
38.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol 66: 571–599, 2004.
39.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene 22: 7537–7552, 2003.
40.Minisclou C, Rouquier L, Benavides J, Scatton B, Claustre Y. Muscarinic receptor-mediated increase in extracellular inositol 1,4,5-trisphosphate levels in the rat hippocampus: an in vivo microdialysis study. J Neurochem 62: 557–562, 1994.
41.Nielsen SP, Petersen OH. Transport of calcium in the perfused submandibular gland of the cat. J Physiol 223: 685–697, 1972.
42.Nikolaev VO, Lohse MJ. Monitoring of cAMP synthesis and degradation in living cells. Physiology Bethesda 21: 86–92, 2006.
43.Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium 38: 161–169, 2005.
44.Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280: 40201–40209, 2005.
45.Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J Biol Chem 275: 3256–3263, 2000.
46.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 5: 1176–1180, 2004.
47.Putney JW Jr. New molecular players in capacitative Ca2+ entry. J Cell Sci 120: 1959–1965, 2007.
48.Qian WJ, Aspinwall CA, Battiste MA, Kennedy RT. Detection of secretion from single pancreatic beta-cells using extracellular fluorogenic reactions and confocal fluorescence microscopy. Anal Chem 72: 711–717, 2000.
49.Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem 232: 1065–1076, 1958.
50.Raya A, Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet 7: 283–293, 2006.
51.Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature 427: 121–128, 2004.
52.Roberts RE, Marsden CA, Kendall DA. Studies of inositol phosphate export from neuronal tissue in vitro. J Neurochem 69: 1291–1299, 1997.
53.Rosenberg PA, Dichter MA. Extracellular cAMP accumulation and degradation in rat cerebral cortex in dissociated cell culture. J Neurosci 9: 2654–2663, 1989.
54.Sager G. Cyclic GMP transporters. Neurochem Int 45: 865–873, 2004.
55.Sorbera LA, Morad M. Modulation of cardiac sodium channels by cAMP receptors on the myocyte surface. Science 253: 1286–1289, 1991.
56.Strewler GJ. Release of cAMP from a renal epithelial cell line. Am J Physiol Cell Physiol 246: C224–C230, 1984.
57.Strouch MB, Jackson EK, Mi Z, Metes NA, Carey GB. Extracellular cyclic AMP-adenosine pathway in isolated adipocytes and adipose tissue. Obes Res 13: 974–981, 2005.
58.Sutherland EW. Studies on the mechanism of hormone action. Science 177: 401–408, 1972.
59.Tang RH, Han S, Zheng H, Cook CW, Choi CS, Woerner TE, Jackson RB, Pei ZM. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science 315: 1423–1426, 2007.
60.Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84: 137–167, 2004.
61.Tepikin AV, Kostyuk PG, Snitsarev VA, Belan PV. Extrusion of calcium from a single isolated neuron of the snail Helix pomatia. J Membr Biol 123: 43–47, 1991.
62.Tepikin AV, Voronina SG, Gallacher DV, Petersen OH. Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J Biol Chem 267: 3569–3572, 1992.
63.Tepikin AV, Voronina SG, Gallacher DV, Petersen OH. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J Biol Chem 267: 14073–14076, 1992.
64.Thimm J, Mechler A, Lin H, Rhee S, Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem 280: 10646–10654, 2005.
65.Thomas AP. Sharing calcium opens new avenues of signalling. Nat Cell Biol 2: E126–E127, 2000.
66.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci USA 103: 5567–5572, 2006.
67.Vendetti S, Patrizio M, Riccomi A, De Magistris MT. Human CD4+ T lymphocytes with increased intracellular cAMP levels exert regulatory functions by releasing extracellular cAMP. J Leukoc Biol 80: 880–888, 2006.
68.Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335: 37–46, 2004.
69.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem 278: 17664–17671, 2003.
70.Zaccolo M, Pozzan T. CAMP and Ca2+ interplay: a matter of oscillation patterns. Trends Neurosci 26: 53–55, 2003.
71.Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol 3: 906–918, 2002.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









