{ DOWNLOAD AS PDF }
 ABOUT AUTHOR
ABOUT AUTHOR
Abhishek Singhal
Department of Pharmacology,
Smt. Kashibai Navale College of Pharmacy, Kondhwa (Bk.),
Pune, Maharastra, India
*abhishekspharma@gmail.com
ABSTRACT
Aim: This review is written to describe the role of immunotherapy in cancer treatment.
Annually, 13,000 deaths result from bladder cancer. Since the first report of intravesical use of BCG in 1976, investigators try to understand the working mechanism of BCG as an antitumour modality. Arbitrarily, BCG therapy consists of a single course of six weekly intravesical instillations. Bacillus Calmette-Gue´rin, an attenuated strain of Mycobacterium bovis, was developed by Calmette and Gue´rin with the intention to generate a vaccine against tuberculosis. Extension of BCG treatment (maintenance immunotherapy) is used to increase efficacy.
Results of various experiments describe that after instillation in the bladder, BCG accumulates near the bladder wall and is internalised and processed by professional antigen-presenting cells (APCs) and (high-grade) tumour cells. Then BCG antigens are presented to CD4+ T cells. Then local synthesis of a particular set of cytokines or cell-mediated immune response. NK cells may be involve in tumour cell killing.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2303
|
PharmaTutor (ISSN: 2347 - 7881) Volume 3, Issue 1 How to cite this article: A Singhal; Role of BCG in Treatment of Bladder Cancer; PharmaTutor; 2015; 3(1); 19-24 |
INTRODUCTION
Intravesical instillation of Bacillus Calmette-Gue´rin (BCG) is used for the treatment of superficial bladder cancer, both to reduce the recurrence rate of bladder tumour and to diminish the risk of progression.[1]
In clinical practice, adjuvant treatment of superficial bladder cancer with Bacillus Calmette-Gue´rin (BCG) is performed by six weekly intravesical instillations of viable BCG after initial transurethral resection of the tumor. BCG therapy is probably the most effective immunotherapy to date, and various immunostimulatory effects of BCG in vitro and in vivo have been described.[2]
BACKGROUND
Bacillus Calmette-Gue´rin, an attenuated strain of Mycobacterium bovis, was developed by Calmette and Gue´rin with the intention to generate a vaccine against tuberculosis.[1]
Since the first report of intravesical use of BCG in 1976, investigators try to understand the working mechanism of BCG as an antitumour modality. Arbitrarily, BCG therapy consists of a single course of six weekly intravesical instillations. Extension of BCG treatment (maintenance immunotherapy) is used to increase efficacy. Despite its success, 30–50% of patients either fail to respond or relapse within the first 5 years of treatment. Unfortunately, BCG, a viable, living organism, can cause infections resulting in side effects ranging from bothersome cystitis in the majority of patients to sepsis eventually leading to death in rare cases. In order to reduce the side effects of BCG and to improve efficacy, interesting approaches are developed, such as the application of a cell wall–DNA complex (MCC) of Mycobacterium phlei, which was effective in patients who failed BCG therapy.[1]
EPIDEMIOLOGY
Annually, 13,000 deaths result from bladder cancer.[3] Bladder cancer is the fifth most common malignant disease, with an incidence that reached >63,000 new cases in the United States alone in 2005. Bladder cancer is detected in 75% of patients at an early, superficial stage.[4]
MECHANISMS FROM VARIOUS RESEARCHES
Nowadays, it is generally assumed that the BCG-induced antitumour activity is critically dominated by a local nonspecific immunological reaction reflecting the activity of immunocompetent cells.
a) BCG immunotherapy and role of urothelial cells:
Internalised BCG increased the production of cytotoxic nitric oxide (NO) in uroyhelial cell carcinoma (TCC) cells. Patients treated with BCG showed an augmented bladder NO production and an upregulation of urothelial-associated nitric oxide synthase. Bacillus Calmette- Gue´rin accumulates near, and adheres to, the bladder wall. After passage through the GAG (glycosaminoglycans) layer, BCG is internalised and processed by professional APC (antigen-presenting cells) and tumour cells. The modified gene expression of these cells accumulates in the secretion of particular cytokines and presentation of BCG antigens. Bacillus Calmette-Gue´rin antigens are presented via MHC class II molecules to CD4þ T cells and via MHC class I molecules to CD8þ T cells. Lipid and glycolipid BCG antigens can be presented to CD4þ and CD8þ T cells in a non-MHC-restricted, CD1-estricted fashion. Production of chemokines, such as IL-8, secreted partly by BCG-internalised tumour cells, contributes to the local activation of the immune system. Consequently, activated leucocytes and mononuclear cells invade the bladder wall. These developments provide the condition for a Th1 response, associated with particular cytokines (IFN-g, IL- 2, IL-12 and TNF-b). This cytokines profile promotes delayed-type hypersensitivity response, cytotoxic cell response, and macrophage activation or cellular immune inflammatory reaction. Depending on bacterial and host components, an upregulation of the Th2 response, associated with cytokines IL-6 and IL-10, may occur to some degree and adversely affect the functioning of the Th1 response. The Th1 cytokine profile enables recruitment and maturation of cytotoxic effector cells.[1] (Figure 1)
[adsense:468x15:2204050025]
b) NK cells are essential for effective BCG immunotherapy:
NK cells represent a distinct subset of lymphocytes and have the ability to kill tumor cells spontaneously without the need for prior activation or MHC class I restriction. Within the scenario of a BCG-induced anti-tumor response, NK cells could become activated by cytokines secreted by macrophages/ dendritic cells (e.g., IL-12) as well as CD8 cells and CD4 cells (e.g., IL-2, IFN-g). After activation, these NK cells would then contribute to the anti-tumor effect as producers of IFN-g (interferon-gamma), and, more likely, as cytotoxic effector cells, which directly lyse bladder tumor target cells.[2] (Figure 1)
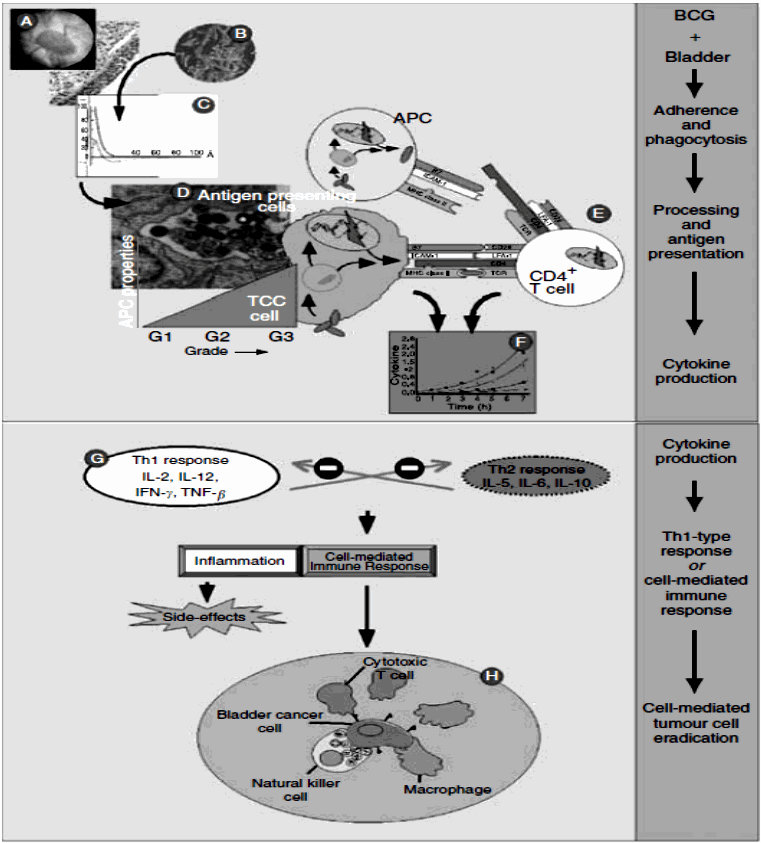
Figure 1 Simplified scheme of the supposed mechanism of action of BCG in tumour cell eradication:[1]
After its instillation in the bladder (A), BCG (B) accumulates near the bladder wall, followed by adherence and passage through the GAG layer of the bladder wall (C). Bacillus Calmette-Gue´rin is internalised and processed by professional antigen-presenting cells (APCs) and (high-grade) tumour cells (D), and BCG antigens are presented to CD4þ T cells (E). Depending on various conditions, this results in the local synthesis of a particular set of cytokines, known as the Th1-type or cell mediated immune response (F, G). The Th1 cytokine profile enables recruitment and maturation of cytotoxic effector cells. No definite statements can be made yet about the actual effector cell(s), but a key role for NK cells in tumour cell killing has been proposed (H).
c) Tumor necrosis factor-related apoptosis-inducing ligand:
BCG instillation resultsin an early granulocytic influx into the bladder wall followed by mononuclear cells where CD4 cells predominate. A proinflammatory Th1 cytokine response predominates (interleukin (IL)-2, IL- 12, IFN-(interferon-gamma)) after BCG stimulation, and the Th1 response is often associated with a favorable response. Moreover, BCG combined with IFN-2B additionally polarizes the Th1 cytokine response (increasing IFN-, IL-12, tumor necrosis factor production; decreasing IL-6, IL-10 production). Upon instillation into the bladder, BCG attachment to the urothelium, via fibronectin, is essential for effective therapy. Viable BCG must be used to induce the antitumor response because heat-killed, nonviable BCG does not bind to fibronectin. An early influx of granulocytes occurs, followed by the influx of mononuclear cells, after BCG is instilled into the bladder, and CD4 and CD8cells are required for BCG-mediated antitumor activity. Interestingly, we observed high TRAIL expression on the neutrophils present in the urine of patients immediately after BCG instillation (3–5 h), suggesting a new and very profound role for components (i.e., the massive granulocyte influx) of the innate immune system in the BCG immunotherapeutic scheme. In addition to the potential predictive value of monitoring the TRAIL response after BCG instillation, the observation of TRAIL dependent killing of bladder cancer cells after BCG instillation has many potential clinical ramifications. Presence of soluble TRAIL/ Apo-2L in the urine of patients after BCG immunotherapy and observed increased levels of TRAIL/Apo-2L in those patients that responded favorably to BCG therapy. TRAIL/ Apo-2L expression on voided neutrophils, which may signal a new and significant role for the massive granulocyte influx after BCG instillation. Collectively, this data suggests TRAIL/Apo-2L has a mechanistic role in BCG immunotherapy.[3]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
d) BCG immunotherapy of bladder cancerand role of Neutrophil granulocytes:
Circulating human PMN (polymorphonuclear neutrophil granulocytes) are a short-lived subpopulation of innate immune cells with a half-life of only 6 to 10 hours after being released from the bone marrow and undergo rapid apoptosis followed by elimination in the liver and spleen. On tissue injury, they home to the site of trauma or infection and migrate from the bloodstream into the inflamed tissue. These activated, infiltrating PMN have a considerably longer life span than their circulating counterparts. They can phagocytose microorganisms, generate reactive oxygen intermediates, and release lytic enzymes with antimicrobial potential. PMN are the first immune cells to leave the blood vessels and infiltrate tissue that has been invaded by mycobacteria.
Following intravesical instillation, BCG provokes an early influx of innate immune cells, such as polymorphonuclear neutrophil granulocytes (PMN) and a proinflammatory cytokine milieu of predominantly TH1 type (5, 8, 9). After repeated instillations, the bladder wall is infiltrated by mononuclear cells, consisting mainly of monocytes/macrophages, CD4+ and CD8+ T lymphocytes, as well as natural killer (NK) cells, which form chronic granuloma-like cellular infiltrates in the suburothelial stroma. Vast numbers of PMN in the urine after BCG administration, shows these cells as a dominating source of early urinary chemokines, such as IL-8. T-cell attraction was due to the combined action of BCG-activated PMN and primed monocytes. In addition to IL-8, GRO-a, and MIP-1a, a significant increase in MIF secretion from BCG-stimulated peripheral blood PMN as well as from urine PMN was dected compared with baseline levels. It has been described to serve as a central regulator of innate and inflammatory immune responses. Thus, it controls the ‘‘set point’’ and magnitude of immune responses by overriding the immunosuppressive effects of glucocorticoids. PMN-derived MIF might also be involved in the control of the BCG-induced inflammatory antitumor response. In summary, it is described first time that BCG-stimulated PMN are an indispensable subset of immunoregulatory cells and orchestrate T-cell chemotaxis to the bladder during BCG immunotherapy. Using functional chemotaxis assays, it could be shown that activated PMN additionally induce monocyte migration and exploit the accessory function of these immune cells to attract T cells.[4]
e) BCG induce macrophage cytotoxicity through th1-stimulating cytokines: Macrophages exhibit multiple pivotal functions in host immune responses. Macrophages act as a first line of defence against microorganism infection in the innate immune system. In the case of BCG infection, anti-mycobacterial immunity develops in the host, which involves the initial ingestion of BCG by immature macrophages, the early growth of BCG in infected macrophages and the late inhibition and destruction of BCG by activated macrophages. Once activated,macrophages gain effector functions and act as inflammatory, microbicidal and tumoricidal cells through cell–cell contact and/or release of soluble factors such as cytotoxic cytokines and nitrate oxide (NO).
The BCG-activated PECs showed potent cytotoxicity and killed MBT-2 cells in a dose-dependent manner. Depletion of T cells, natural killer (NK) cells, or both, in PEC preparations exhibited a marginal or small reduction of MBT-2 cell killing, suggesting that macrophages played a primary role in PEC cytotoxicity. Results suggested that Th1- stimulating cytokines play an important role in BCG-induced macrophage cytotoxicity and that combination of BCG with selected Th1-stimulating cytokines, either supplemented or expressed by BCG, may enhance the effect of BCG in the treatment of bladder cancer patients. The same observation also suggests that BCG-activated macrophages are particularly effective at promoting cytotoxic T cell responses. In addition to their cytotoxic activities, both T cells and NK cells could also have played an important role in amplifying macrophage cytotoxicity. IL-12, IL-18 and TNF-a, the major cytokines produced predominantly by activated macrophages, are known to enhance BCG for induction of IFN-g from T cells and NK cells, which in turn could feed back positively to augment macrophage phagocytosis and production of soluble cytotoxic factors.[5] (Figure 1)
f) BCG induce Surface antigen expression on bladder tumor cells: Most effective route of administration of BCG is direct intratumoral injection and that direct contact between BCG and the tumor cells is an essential initial step in the antitumor activities of BCG. BCG induced the augmented expression of surface antigens, such as MHC Class II, CD1, CD80 and ICAM-1, of bladder tumor cells. Furthermore, BCG-treated MBT-2 cells (Murine Bladder Tumour) could stimulate BCG-sensitized lymphocytes to produce IL-2 and IFN-g (Interferon g). These results strongly suggest that bladder tumor cells gained the characteristics and functions of antigen-presenting cells (APC). MHC (Major Histocompatibility Complex) Class II antigens serve as restriction elements for cells that present antigens to CD4+ helper T cells, and it has been reported that intravesical BCG treatment induces the expression of MHC Class II on normal urothelium and bladder tumor cells in patients.[6]
First time reported, that the expressions of MHC Class II, CD1, CD80 and ICAM-1 (Intercellular Adhesion Molecule-1) were augmented directly by BCG in vitro, not via the host immune mechanisms.
g) BCG exhibit changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-L):
Sequential instillations of BCG induced secretion of IL-1/3, IL-2, IL-6, IL-8, IL-10, TNF-a (tumour necrosis factor), IFN-7 (interferon-gamma) and sICAM-1 into urine. Cytokine levels were initially low and increased with repeated instillation of BCG. IL-1/3, IL-6, IL-8, IL-10 and ICAM-1 were detected after the first instillation. IL-2, TNF-a and IFN-7 were not detected until later instillations. IL-4 was not detected in any patients' urine. Soluble TNF receptors (types I and II) excreted after BCG therapy, shown that the TNF present in urine is biologically inactive, possibly due to complexing with soluble receptor.[7]
DISCUSSION
All the researches show the role of BCG in treatment of urinary bladder cancer. As BCG stimulate microphase cytotoxicity through th-1 stimulating cykines. BCG induce surface antigen expression on tumour cells of bladder. Urothelial cells and neutrophil granulocytes and NK cell play important role in BCG immunotherapy. BCG exhibit changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-l). BCG play important role in tumor necrosis factor-related apoptosis-inducing ligand. BCG therapy exhibit cytotoxicity through various cytokines and mediators. So BCG immunotherapy effective against bladder cancer.
CONCLUSION
Intravesical BCG is the most effective therapy for CIS and superficial cancer of bladder. It provides a superior protection from tumor recurrence and even reduces disease progression. Dose reduction of BCG has been associated with a corresponding reduction in BCG-related toxicity. However, at present most authorities recommend standard dose (80 mg) therapy. Six-week induction therapy is standard of care to prevent recurrence; however, maintenance with BCG immunotherapy for at least one year is advocated to prevent tumor progression.
REFERENCES
1. Bevers, R.F.M. et al., Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. British Journal of Cancer, 2004, 91, 607–612.
2. Brandau, S. et al., NK cells are essential for effective bcg immunotherapy. Int J Cancer, 2001, 92, 697–702.
3. Ludwig, A. T. et al., Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand: A Novel Mechanism for Bacillus Calmette-Guérin-Induced Antitumor Activity. Cancer Res, 2004, 64, 29–35.
4. Suttmann, H. et al., Neutrophil granulocytes are required for effective bacillus calmette-guérin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res, 2006, 66, 8250-8257.
5. Luo, Y. et al., Role of Th1-stimulating cytokines in bacillus Calmette–Guérin (BCG)-induced macrophage cytotoxicity against mouse bladder cancer MBT-2 cells. Clinical and Experimental Immunology, 2006, 146, 181–188.
6. Ikeda, N. et al., Surface antigen expression on bladder tumor cells induced by bacillus Calmette-Guérin (BCG): A role of BCG internalization into tumor cells. International Journal of Urology, 2002, 9, 29–35.
7. Jackson, A.M. et al., Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-l) in bladder cancer patients after Bacillus Calmette-Guerin (BCG) immunotherapy. Clin Exp Immunol, 1995, 99, 369-375.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









