{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Patel Divya A*., Varodiya Priyanka S., Raj Hasumati A.
Department of Quality Assurance, Shree Dhanvantary Pharmacy College,
Kim, Surat, Gujarat, India
divyapatel388@gmail.com, drharaj@yahoo.com
ABSTRACT
This review article presents the pharmacology of combined edaravone and argatroban therapy especially in acute ischemic stroke. Edaravone (MCI-186) is a free radical scavenger, a novel neuroprotective agent. Argatroban is a selective thrombin inhibitor.The antithrombotic agent was used in acute cerebral infarction. If the antithrombotic agent is administered in large quantities, the condition of patient become worse by occurrence of adeverse effect of cerebral haeorrhage. The use of edaravone in combination with antithrombotic agent has been proved to provide beneficial effect in acute ischemic stroke as edaravone has no influence to coagulation of blood and platelets aggregation. The combination therapy has fewer hemorrhagic adeverse effect. The mechanism of argatroban and edaravone is quite different. Argatroban, an anti-coagulant drug, directly improves the microcirculation of ischemic brain tissue while edaravone could indirectly attenuated brain edema by protection of endothelial cells damaged by free radicals generated after ischemic insult. The combination of both would have reciprocal and enhanced neuroprotective effects against ischemic insult. Both the drugs were approved by Japanese government and has been used in acute brain infarction in japan. The main objective of this review article is to provide pharmacological information of combined therapy of edaravone and argatroban to researcher in development of combined dosage form of this.
REFERENCE ID: PHARMATUTOR-ART-2269
INTRODUCTION
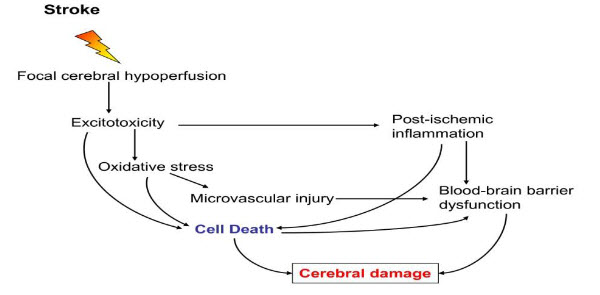
Figure 1: Mechanism of stroke[1]
I) OXIDATIVE STRESS [1]
Oxidative stress is the pathogenesis of a number of neurological conditions including stroke. Oxidative stress is condition when the physiological balance between oxidants and antioxidants is disrupted. Oxidative stress leads to the formation of reactive oxygen species, reactive nitrogen species through multiple injury mechanisms, such as mitochondrial inhibition, Ca2+ overload, reperfusion injury, and inflammation. Most of ROS are generated during an acute ischemic stroke and that oxidative stress is an important mediator of tissue injury in acute ischemic stroke. Brain ischemia generates superoxide (O2-), which is the primary radical from which hydrogen peroxide is formed. Hydrogen peroxide is the source of hydroxyl radical (OH). Thus, free radicals are regarded as an important therapeutic target for improving the condition ischemic stroke.
II) POST ISCHEMIC INFLAMMATION [1]
Microglia can transform into phagocytes, after activation by ischemia, and they can release a variety of substances many of which are cytotoxic and cytoprotective. Microglia may exert neuroprotection by producing neurotrophic molecules such as brain-derived neurotrophic factor(BDNF), insulin- like growth factor I (IGF-I), and several other growth factors. The activated microglial cells in response to ischemia have the potential of releasing several pro-inflammatory cytokines such as TNF- α, IL-1β, and IL-6, as well as other potential cytotoxic molecules including NO, ROS, and prostanoids.
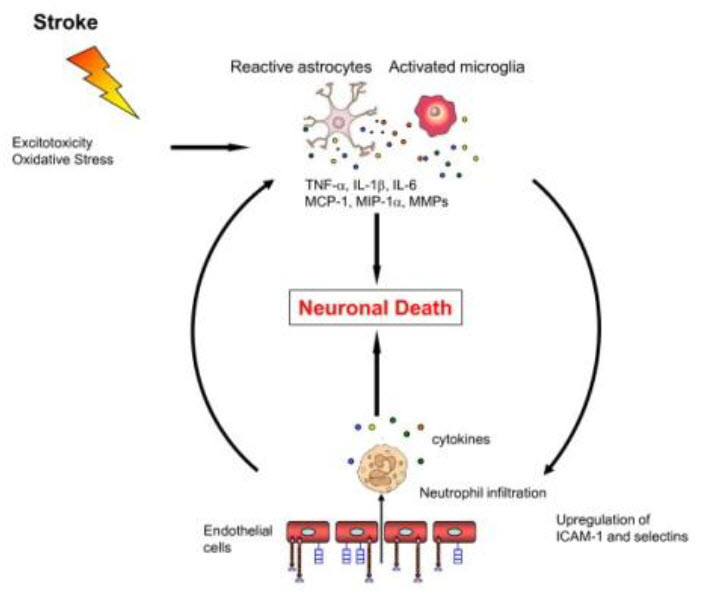
Figure 2: Mechanism of oxidative stress [1]
EDARAVONE
Chemical name:- 3-methyl-1-phenyl-2 –pyrazolin-5-one. Also known as MCI-186.
A pyrazole derivative appears as white to off white crystalline powder. The drug is slightly soluble in Distilled Water. (3 g/L). Freely soluble in methanol.
Edaravone melts at 127-131 ºC [2,3]
The pKa value of edaravone is 7.0.
Edaravone and its derivatives were synthesized by condensation of hydrazine and 3-oxopropionic acid esters in refluxed ethanol. 2-Pyrazolin-5- one was selected as the scaffold, (a) modification of the phenyl group at the 1-position, (b) introduction of substituents on the 1-phenyl moiety, (c) modification of the methyl group at the 3 position, and (d) introduction of substituents on the methylene moiety at the 4-position.
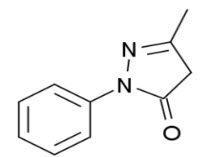
Figure 3: The chemical structure of edaravone [2]
This optimization suggested that compounds possessing lipophilic substituents were potent inhibitors of lipid peroxidation. The electronic properties of the substituents did not affect the activity of these compounds. The absence of inhibitory activity in 1-phenyl- 3,4,4-trimethyl-2-pyrazolin-5-one, which is not a subject to keto-enol tautomerization, [4]

Figure 4: Design of phenol like compound and its tautomerization [4]
Edaravone has three tautomeric forms: the amine, keto, and enol forms (Fig. 5). Approximately 50% of edaravone exist in an anionic form at physiological pH; this form may react strongly with reactive oxygen species in the brain. Compound which generate aromatic hydroxyl group by keto enol tautomerization would show radical scavenging and antioxidant activity and would not have toxicities of phenol.
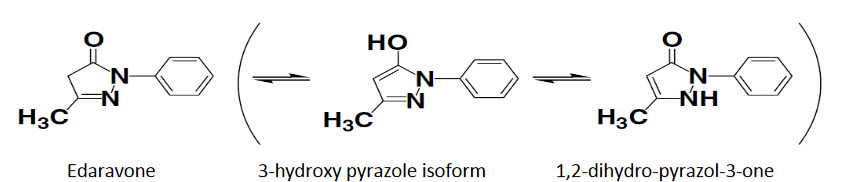
Figure 5: Tautomeric forms of edaravone [4]
i) IN VITRO PHARMACOLOGY:
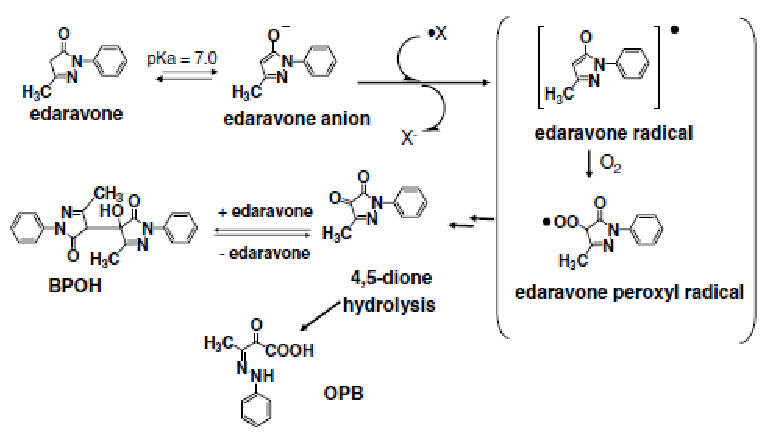
Figure 6: In vitro pharmacology of edaravone [4]
An electron transfer from edaravone anion yields radical derived anion and edaravone radical, which reaction breaks the chain of oxidation of lipid. Edaravone radical is transformed in to 4,5-dione via edaravone peroxyl radical with reaction of molecular oxygen.
ii) IN VIVO PHARMACOLOGY:
The preventive effect is due to suppression of reperfusion caused oxidative endothelium cell damage since edarevone was found to inhibit 15-HPETE (15-hydroxy peroxyicosatetraenoic acid) as well as cortical edema of rat brain induced by arachidonate micro injection.
The protective effect of edaravone on brain ischemia has been evaluated by using rodent focal ischemia model with or without reperfusion. Edaravone inhibit the free radicals which are generated during ischemic insult thus provide neuroprotection.
PHARMACOKINETIC
Edaravone is excreted as unmetabolized drug (~1%).
Metabolized by sulfation (5–13%) or glucuronidation (68–83%) and excreted in urine within 24 hours of administration.
Edaravone is a neuroprotective agent used for aiding neurological recovery in acute brain ischemia and cerebral infarction.[5] It acts as a potent antioxidant and strongly scavenges free radicals, protecting against oxidative stress and neuronal apoptosis.[6-8] Edaravone attenuate methamphetamine- and 6-OHDA-induced dopaminergic neurotoxicity in the striatum and substantianigra, and does not affect methamphetamine-induced dopamine release or hyperthermia.[9,10] It has also been demonstrated to protect against MPTP-mediated dopaminergic neurotoxicity to the substantianigra, though notably not to the striatum.[11-13]
ARGATROBAN [14]
Argatroban is also known as argipidine.
Argatroban is a synthetic direct thrombin inhibitor derived from L-arginine.
The chemical name for Argatroban is 1-[5[(aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]amino]pentyl]-4-methyl-2piperidinecarboxylic acid, monohydrate.
Argatroban is a white, odorless crystalline powder that is freely soluble in glacial acetic acid, slightly soluble in ethanol, and insoluble in acetone, ethyl acetate, and ether.
The molecular formula of Argatroban is C23H36N6O5S•H2O. Its molecular weight is 526.66 g/mol. The structural formula is shown below:
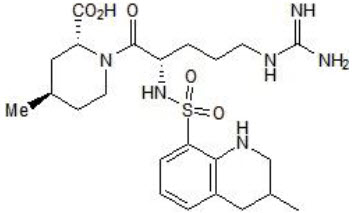
Figure 7: The structure of argatroban
Argatroban consists of a mixture of R and S stereoisomers at a ratio of approximately 65:35. Argatroban has 4 asymmetric carbons. One of the asymmetric carbons has an R configuration (stereoisomer Type I) and an S configuration (stereoisomer Type II).
MECHANISM OF ACTION[14]
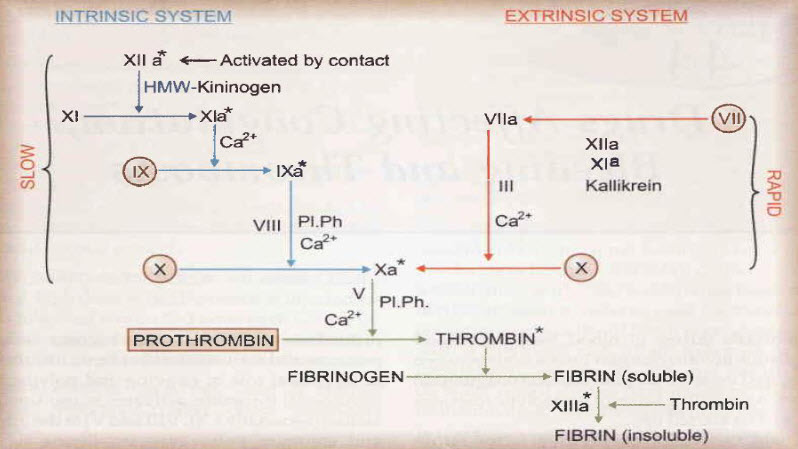
Figure 8: Coagulation cascade [15]
Due to its selective inhibitory mechanism, argatroban blocks both circulating and clot-bound thrombin. Argatroban is a direct thrombin inhibitor that binds reversibly to the catalytic site of thrombin and that does not require other cofactors to exert its antithrombotic action. A rapid onset of its anticoagulant action is achieved after intravenous administration. The elimination half-life of argatroban is (52+/-16 minutes) which is short enough to ensures a rapid restoration of hemostasis upon cessation of treatment. Argatroban produces a predictable dose response, and its anticoagulant actions can be monitored easily through the routine coagulation tests activated partial thromboplastin time (aPTT) and activated clotting time (ACT). The specific mechanism of action of argatroban indicate that it could be beneficial in all indications where other intravenous anticoagulants are used. [16]
PHARMACOKINETICS [16]
Distribution: Argatroban distributes mainly in the extracellular fluid. An apparent steady-state volume of distribution of 174 mL/kg (12.18 L in a 70-kg adult). Argatroban is 54% bound to human serum proteins, with binding to albumin and α1-acid glycoprotein being 20% and 34%, respectively.
Metabolism:
The elimination half-life of Argatroban ranges between 39 and 51 minutes. There is no interconversion of the 21–(R):21–(S) diastereoisomers. The plasma ratio of these diastereoisomers is unchanged by metabolism or hepatic impairment, remaining constant at 65:35 (± 2%).
The main route of Argatroban metabolism is hydroxylation and aromatization of the 3-methyltetrahydroquinoline ring in the liver. The primary metabolite (M1) exerts 3- to 5-fold weaker anticoagulant effects than Argatroban. Unchanged Argatroban is the major component in plasma. The plasma concentrations of M1 range between 0% and 20% of that of the parent drug. The other metabolites (M2 to M4) are found only in very low quantities in the urine and have not been detected in plasma or feces. The formation of each of the 4 known metabolites is catalyzed in vitro by the human liver microsomal cytochrome P450 enzymes CYP3A4/5.
Total body clearance is approximately 5.1 mL/kg/min (0.31 L/kg/hr) for infusion doses up to 40 mcg/kg/min.
Excretion: Argatroban is excreted primarily in the feces, presumably through biliary secretion.
COMBINATION THERAPY OF EDARAVONE AND ARGATROBAN [17]
The combination provides the remedy for prevention of ischemic diseases with less adeverse effect , safety and having high clinical effects.[18]
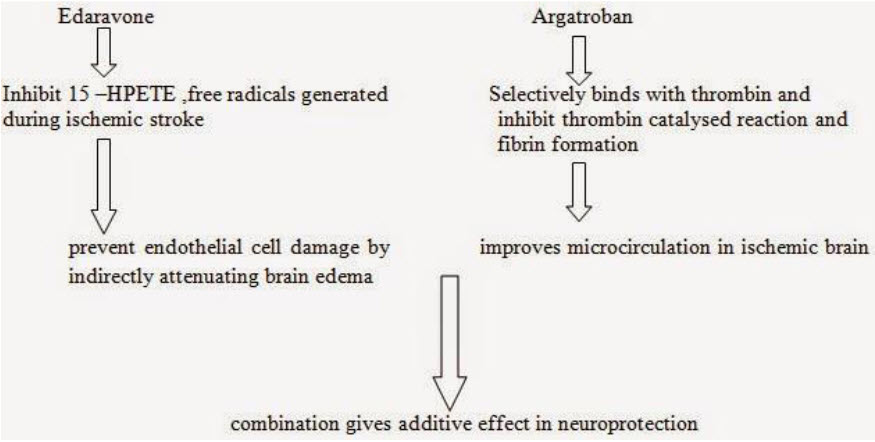
a combination of the selective thrombin inhibitor, argatroban, and the free radical scavenger, edaravone (MCI-186), attenuates postischemic hypoperfusion and decreases mortality after 15 min of forebrain ischemia in the gerbil. Argatroban or edaravone alone significantly increased postischemic cerebral blood flow and attenuated brain edema after reperfusion. However, only the combination increased the survival ratio (PB/0.05 by Mantel-Cox) and protected the damage of neuronal cells. The anticoagulants and free radical scavengers reciprocally function to inhibit the progression of ischemic cell damage and that a combination of these types of drugs will help to improve the condition after cerebral ischemia.
The combination provides the remedy for prevention of ischemic diseases with less adeverse effect , safety and having high clinical effects.[18]
Both the drugs were approved by the Japanese Government, and have frequently been used for the treatment of acute brain infarction in Japan. This study will test the safety and efficacy of the combination therapy with these agents in patients with acute non-cardioembolic and non-lacunar ischemic stroke.[19]
MECHANISM OF ACTION OF COMBINED THERAPY[18,19]
CONCLUSION
By reviewing the all literatures, the combination therapy was found to be effective in treatment of acute ischemic stroke. This review represents individual pharmacology and pharmacokinetic of edaravone and argatroban as well as mechanism of action of combination of edaravone and argatroban in treatment of acute ischemic stroke. This review will helpful for researcher in future study and also for development of combined formulation of edaravone and argatroban as there no formulation is available.
REFERENCES
1. Shaheen E Lakhan, Annette kirchgessner and Magelalena Hofer ; Review IInflammation mechanism in ischemic stroke : therapeutic approaches;Journal of Translational medicines; Nov, 2009, 7-97.
2. Edaravone Drug Info.(database available on internet):Chemical Book. Available from: chemicalbook.com/ProductMSDSDetailCB1287462_EN.htm
3. Edaravone Drug Info.(database available on internet):Lookchem. Available from: lookchem.com/Edaravone/
4. Toshiaki W, Munenori T, Satoru T. The Novel Antioxidant Edaravone: From Bench to Bedside. Cardiovascular Therapeutics; 20098, 26: 101–114.
5. Doherty AM. Annual Reports in Medicinal Chemistry. Boston: Academic Press; 2002, 37.
6. Watanabe T. et al. Research and development of the free radical scavenger edaravone as a neuroprotectant. Yakugaku Zasshi (in Japanese); 2004, 124(3): 99–111.
7. Higashi Y, Jitsuiki D, Chayama K, Yoshizumi M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Patents on Cardiovascular Drug Discovery; 2006, 1(1):85–93.
8. Yoshida H. et al.Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Reviews; 2006, 12(1):9–20.
9. Yuan WJ. et al. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neuroscience; 2008,9:75.
10. Kawasaki T. et al. Protective effect of the radical scavenger edaravone against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. European Journal of Pharmacology; 2006, 542(1-3): 92–99.
11. Kawasaki T. et al. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a radical scavenger, prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced neurotoxicity in the substantianigra but not the striatum. The Journal of Pharmacology and Experimental Therapeutics; 2007, 322(1):274–281.
12. Yokoyama H. et al. Role of reactive nitrogen and reactive oxygen species against MPTP neurotoxicity in mice. Journal of Neural Transmission; 2008, 115(6):831–842.
13. Yokoyama H. et al. Comparative pharmacological study of free radical scavenger, nitric oxide synthase inhibitor, nitric oxide synthase activator and cyclooxygenase inhibitor against MPTP neurotoxicity in mice. Metabolic Brain Disease; 2008, 23(3):335–349.,
14. Available from : gsksource.com/gskprm.
15. Tripathi.K.D , Essential of medical pharmacology ; 6 th edition ;594
16. Escolar G, Bozzo J, Maragall S; Argatroban a direct thrombin inhibitor with reliable and predictable anticoagulant action; Drug today 2006, Apr; 42(4), 223-236
17. Young-Jian Jin, Tatsuo Mimma, Valerica Raicu, Kae Chang Park, Keiji Shimizu; Combined argatroban and edaravone caused additive neuroprotection against 15 min of forebrain ischemia in gerbils; Neuroscience Research 43(2002) 75-79.
18. Keiichi Tesseikai, Katsumi C/o; Drugs comprising combination of anti thrombotic agent with pyrazolone derivative; Patent EP1437137B1.
19. Combination therapy for Acute ischemic stroke study group; Edaravone and argatroban stroke therapy study for acute ischemic stroke; clinicaltrials.gov.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 11 Received On: 26/08/2014; Accepted On: 07/09/2014; Published On: 01/11/2014How to cite this article: DA Patel, PS Varodiya, R Hasumati; A Review on Pharmacology of Combined Edaravone and Argatroban Therapy in Acute Ischemic Stroke; PharmaTutor; 2014; 2(11); 42-49 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









