ABOUT AUTHORS:
Jyoti Malik
Hindu College of Pharmacy,
Sonepat, Haryana
jyotimalik127@gmail.com
ABSTRACT:
There was a need for delivery systems that could maintain a steady release of drug to the specific site of action. Therefore, drug delivery systems were developed to optimize the therapeutic properties of drug products and render them more safe, effective, and reliable. In comparison with many of the other drug delivery systems, implantable pumps and implants for variable rate delivery are at a crude stage of development. Although the typical implantable pump consists of different mechanisms to regulate drug delivery. The benefits most often provided by the dosage form are expected to be 1) Implantable devices allow site specific drug administration where the drug is needed most. Examples include implants used in the treatment of brain tumors or prostate cancer. This may also allow for significantly lower doses of the drug, which can minimize potential side effects. 2)Implantable devices allow for sustained releaseby the zero-order release rate of a therapeutic agent. The major advantages of these systems contain targeted local delivery of drugs at a constant rate, fewer drugs required to treat the disease state, minimization of probable side effects, and better efficacy of treatment. Due to the development of such sustained release formulations, it is now possible to administer unstable drugs once a week to once a year that in the past required frequent daily dosing.
REFERENCE ID: PHARMATUTOR-ART-2020
INTRODUCTION:
Drug development involves discovering, designing or modifying molecules to maximize desirable therapeutic characteristics while minimizing untoward side effects. Orally administered drug must be protected against denaturation in the gastrointestinal tract and should be capable of absorption across the wall of the stomach or the intestine. After absorption and upon reaching the portal circulation, it must be resistant to hepatic enzymes. The rate of drug absorption and elimination should ensure the blood levels within the therapeutic range. Moreover, the amount of intact drug that reaches the site of action should be sufficiently large to obtain desired therapeutic effect but insufficient to cause untoward side effects. Implantable drug delivery devices are devoid of aforementioned limitations associated with oral, intravenous, topical drug administration vis-à-vis subcutaneously implantable drug delivery devices offer one unique advantage of a retrievable mechanism.
ADVANTAGES:
Convenience: Effective concentration of drug in the blood can be maintained for longer period of time by techniques such as continuous intravenous infusion or repeated injections.Implantation treatment permits patients to get medication outside the hospital setting with marginal medical observation.
Compliance: By reducing the frequency of drug administration over the entire period of treatment improve patient compliance.Patient can forget to take a medicine, but drug delivery from an implant is not dependent of patient input.
Stability: Implants are environmentally stable they should not breakdown under the influence of heat,light,air and moisture.It should be stable and safe and have good mechanical strength.
Improved drug delivery: The drug is distributed locally or in systemic circulation with least interference by metabolic or biological barriers. For example, the drug moiety bypassed the GIT and the liver. The by-passing effect is beneficial to drugs, which are either easily inactivated or absorbed poorly in the GIT and/or the liver before systemic distribution.
controlled release: Implants are available which deliver drugs by zero order controlled release kinetics.So that dosing frequency is reduced, and patient compliance is increased. It leads to enhanced effectiveness and reduce side effects.
Flexibility: In the choice of materials, methods of manufacture,degree of drug loading, drug release rate etc. considerable flexibility is possible. From a regulatory viewpoint, it is regarded as a new product and can lengthen the market protection of the drug.
DISADVANTAGES:
Invasive: To initiate therapy either a minor or a major surgical procedure is required to initiate therapy. Appropriate surgical personnel is required for this, and may be time-consuming. This causes some scar formation at the site of implantation and surgery related complications in a very small number of patients. Uncomfortable feeling for the patient wearing the device.
Danger of device failure: There is no associated danger with this treatment that the device may for some reason fail to work. This again requires surgical involvement to correct.
Termination: Osmotic pumps and non-biodegradable polymeric implants also are surgically recovered at the end of therapy. Although surgical recovery is not required in biodegradable polymeric implants. Its on-going biodegradation makes it difficult to end drug delivery, or to maintain the accurate dose at the end of its lifetime.
Limited to potent drugs: In order to minimize patient’s discomfort the size of an implant is usually kept small. Therefore most implants have a limited loading capacity so that frequently only somewhat potent medicines such as hormones may be appropriate for delivery by implantable devices.
Biocompatibility issues: Concerns over body reactions to a foreign substance often increase the issues of biocompatibility and safety of an implant.
Possibility of adverse reactions: A high concentration of the drug delivered by an implantable device at the implantation site may produce adverse reactions.
MECHANISMS:
* DIFFUSION CONTROLLED: Reservoir type of system consists of a core of drug surrounded by a polymer and diffusion of the drug across the polymer layer is the rate limiting step.Zero order release kinetic easily obtained. In Matrix type systems zero order release rate can be achived by compensating for the increase diffusional distance with an increasing area of the drug.
* CHEMICALLY CONTROLLED: The drug is distributed uniformly throughout the bioerodible polymer,which erodes and decreases in geometry with time to allow the drug release.Zero order kinetics can be achieved if the surface area remains unchanged with time.
* SWELLING CONTROLLED: In this type of system the release rate is equal to the product of surface area and a rate constant corresponding to rate of advance of boundary separating the outer shell from central core.
* OSMOTACALLY CONTROLLED: The system in the form of matrix where the core is surrounded by semipermeable film utilizes osmotic pressure as driving force for delivery of drugs.
* MAGNETICALLY CONTROLLED: This type of system consists of drug and small magnetic beads uniformely dispersed within a polymer matrix.In contact with aqueous media, drug is released in diffusion-controlled manner.
IMPLANTABLE DRUG DELIVERY DEVICES:
1. Field of Controlled Drug Delivery
1.1Transdermal Patches: Transdermal patches generally have hollow microneedles made of a biocompatible polymer through which the drug is delivered below the skin.Transdermal patches have numerous advantages compared with other systems of drug delivery:the drugs are not degraded in the GIT, they are painless,and they deliver a constant dosage without the need for patient’s compliance.
1.2 Polymer Implants: Polymer implants are biodegradable polymers loaded with the drug molecules. The polymer degrades when it comes in interaction with body fluids and in the process releases drug molecules. The rate of degradation of the polymer, and hence the drug
release, can be optimized by modifying the properties of the polymers. The polymer material which are most widely used for these application include, but are not restricted to, Polyglycolic acid(PGA), Polylactic acid(PLA), Polyurethane and the combinations of these in different proportions.
1.3 Bioadhesives:Bioadhesives are substances which form bonds with biological surfaces. The most common substances which are used in this case are polymer hydrogels. The principle of operation is similar to polymer implants in this that they too are loaded with drugs and release drugs at a specific rate when in contact with body fluids.
1.4 Microencapsulation:Microencapsulation refers to the method of covering the drug molecule with a material which will prolong the time before the drug is resorbed, so that it
will remain in the viable state and will be released when it reaches the intended destination. There are variety of ways in which microencapsulation is done. Some of them are use of polymer microspheres, liposomes, nanoparticles etc.
2. Implantable Infusion Pump Systems:
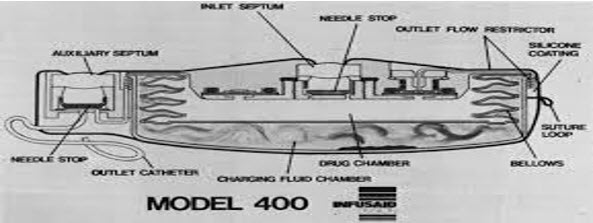
2.1 Vapour pressure powered pimp: This device consist of two chamber and is disk shaped with an inexhaustible volatile liquid power sourse.It is based on the principle that at a given temperature,a liquid in equilibrium with its vapour phase exerts a constant pressure that is endependent of enclosing volume.The fluid which provide appropriate vapour pressure is sealed in a chamber. The drug to be infused is filled in second chamber.The two chambers are separated by flexible metal bellows.After implantation,the volatile liquid vaporizes at body temperature and creates vapour pressure that compress the bellows and expel the infusate through series of flow regulators.
2.2 Osmotic pressure powered pump:
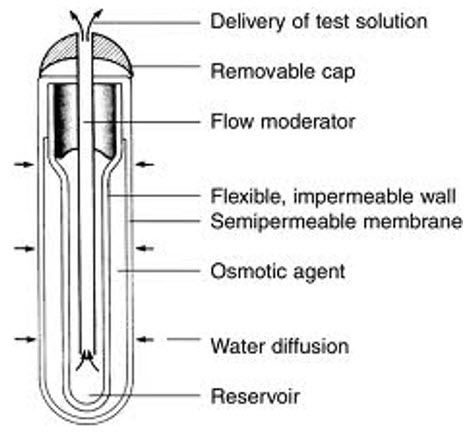
2.2.1 ALZET osmotic pump:These are capsular in shape and made of three layers.The innermost drug reservoir contained in a collapsible impermeable polyester bag.An intermediate slleve of dry osmotic energy source and the outermost rigid, rate-controlling semipermeable membrane fabricated from cellulosic polymer.After implantation, water from surrounding tissue fluids is imbibed through the SPM that dissolve osmagent creating osmotic pressure.The osmotic sleeve thus expands, it squeezes inner flexible drug reservoir and drug solution is expelled in constant volume.The drug delivery continues until the reservoir is completely collapsed.
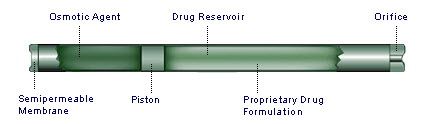
2.2.2 DUROS infusion implant: In Duros system,the outer titanium alloy cylinder is capped by SPM at one end and by an exit port at other end. Within the cylinder are an osmotic engine,a piston and the drug reservoir. After implantation, water from surrounding tissue enters one end of the cylinder through the SPM, causing osmotic engine to swell. This osmotic engine displaces a piston, causes the drug formulation to be released from a port at the other end of the system.
THERAPEUTIC APPLICATIONS:
1.)Ocular disease: Numerous different implantable systems have been estimated to deliver sustained ocular delivery. These comprise membrane-controlled devices, implantable infusion systems and implantable silicone devices.Ocular insert (ocusert)having pilocarpine base and alginic acid in a drug reservoir surrounded by a release-rate controlling ethylene-vinyl acetate membrane is an example of the membrane-controlled system.
2.)Contraception:Norplant a sub-dermal implant for long-lasting transport of the contraceptive agent levonorgestrel recently been approved for marketing by the FDA. The device consists of six silicone membrane capsules each having about 36 mg of levonorgestrel. The capsules are placed sub-dermally on the inside of the upper arm or the forearm in a fan-shaped pattern through a trocar from a single trocar entry point.
3.)Dental application:For numerous dental applications including local prolonged administration of fluoride antibacterial andantibiotics,polymeric implants have been evaluated.Stannous fluoride was integrated into different dental cements for sustained release fluoride delivery.
4.)Immunization: Polymeric implants are being evaluated for better immune response to antigens. The concept here is to offer pulsatile or continuous administration of theantigen over a prolonged period of time. Wise et al.evaluated immunization efficiency of ethylene-vinylacetate copolymer pellets having bovine serum albumin as model antigen.
5.)Cancer: Silicone rod implants analogous to those used for delivery of levonorgestrone have been evaluated for delivery of ethinylestradiol or testosterone propionatein persons with prostate cancer. Lupron depot produced by Takeda chemical industries is an implantation system providingone month depot release of leuprolide acetate, a synthetic analogue of the gonadotropin-releasing hormone (GhRH).
6.)Narcotic antagonists: Naltrexone has been comprehensively evaluated in implant from long term delivery of narcotic antagonists. Naltrexone freebases its hydrochloride or the pamoate acid salt has been formulated in a various polymers and dosage forms for prolonged narcotic antagonist activity.
CONCLUSION:
Site-specific, controlled release of therapeutic agents represents an attractive option for companies looking to enhance the efficacy of a drug product or provide additional benefit in conjunction with an implantable device.Biodegradable polymeric materials provides options for delivery of potent compounds such as hormones, opioids, antibiotics, and oncology drugs. Implanted drug delivery technologies have ability to reduce the frequency of patient driven dosing and to deliver the compound in targeted manner. These issue should ensure that the current high level of interest in this area will extend well into the future and result in significant advances in the field of controlled drug delivery.
REFERENCES:
1. ChienYie W; Novel Drug Delivery Systems, Marcel Dekker Inc.;1992, 2nd Ed, 269.
2. Vyas SP and KharRoop K; Controlled Drug Delivery Concepts and Advances, Vallabh Prakashan (Delhi);2008, 1st Ed, 450-459.
3. Langer R; Where a Pill Won’t Reach,ScientificAmerican; 2003, 288(4): 50– 57.
4. Carmichael M; The Changing Science of Pain,Newsweek; June 4, 2007, 40-47.
5. Vipul R; Vipul's Lifetime Lifeline Permanent Pacemaker and Implantable Cardioverter Defibrillator, U.S. Patent; Jul. 3, 2007, Patent No. 7239917.
6. Sefton MV; Implantable Pumps, CRC Crit. Rev. Biomed. Eng.; 1987, 14: 201–240.
7. Hildgen P, McMullen JN; A New Gradient Matrix: Formulation and Characterization, J. Controlled Release; 1995, 34: 263–271.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









