{ DOWNLOAD AS PDF }
ABOUT AUTHORS
G.Hema Latha1*, MD.Sultan Ali2, C.Vijaya lakshmi R.Kiran kumar1, C.V.H.Hemavathy1
1 Kottam Institute of Pharmacy, Erravally X Roads, Mahaboob Nagar, Telangana
2 Safa College of Pharmacy, Kurnool, A.P
*hemarayudu19@gmail.com
ABSTRACT
Protective effect of ziziphus jujuba in cerebral ischemia reperfusion injury in albino rats. The main aim of the present work is to evaluate the Protective effect of Ziziphus jujuba in cerebral ischemia reperfusion injury in albino rats. Objectives are to test the Preliminary phytochemical screening of Aqueous & Petroleum ether extracts of “Ziziphus Jujuba”. Group 1: Control group; Group 2: Animals treated with lead 0.1 ml/100 g body weight i.p; Group 3: Animals treated with Petroleum ether extract of Ziziphus Jujuba 250mg/kg; Group 4: Animals treated with Petroleum ether extract of Ziziphus Jujuba 500mg/kg.All the groups were subjected to pre-treatment for a period of 7 days except Control group. To compare the changes in Quantification of infract size in normal and treated groups. To compare the changes in SGOT levels in Control and treated groups. The protective effect of the aqueous extract ,petroleum ether extract of Ziziphus Jujuba may be due to the presence of flavonoids, saponins, triterpenoids and tannins. There was a dose dependent increase in cerebral protection in terms of reduction of infarct size of brain tissue and SGOT levels in serum. Furthermore investigation is needed to find out the particular constituent which is responsible this protective activity.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2433
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 9 Received On: 01/03/2016; Accepted On: 25/04/2016; Published On: 01/09/2016 How to cite this article: Latha GH, Ali MS, Lakshmi CV, Kumar RK, Hemavathy CVH; Protective effect of Ziziphus Jujuba in Lead induced Cerebral Ischemia Reperfusion Injury in Albino Rats; PharmaTutor; 2016; 4(9); 40-45 |
INTRODUCTION
Cerebral ischemic [1] disease has become a worldwide health problem affecting all groups of society. Research is underway in an effort to develop pharmacological means to control morbidity and mortality arising from cerebral ischemic disease. Various surgical interventions like carotid endarterectomy[2] are used to re-establish the blood flow to the ischemic brain tissue. This causes a progressive destruction of cells, leading to tissue dysfunction and infarction.[3] This injury is known as cerebral ischemic-reperfusion injury [8]. Ischemia is defined as a reduction in cerebral blood flow sufficient to alter cerebral function. Certain cerebral neurons are selectively vulnerable to ischemia, as in hippocampal CA1 cells compared with dentate granule cells and cerebral neurons [9] and compared with brain stem neurons. Infarction is a histological finding applied to a region of brain that has been injured by ischemia. The place of stroke in animal model is defined by this region, the volume of stroke is measured by integrating areas of infarction over multiple brain slices. Symptoms of stroke includes vertigo, sensory loss, nystagmus, anopia, facial numbness, ataxia, disphagia, dysarthria, ophthalmoplegia, hemiparesis, arm and leg paralysis, amnesia, color amnesia, abulia, [10] alexia, [11] urinary incontinence or coma, depending on arterial territory involved. Major disability is loss of ability to communicate, ambulate, coordinate and reason. High risk factors are identified as the probable cause for ischemia .
AIM & OBJECTIVE
The main aim of the present work is to evaluate the Protective effect of Ziziphus jujuba in cerebral ischemia reperfusion injury in albino rats.Objectives are
1. to test the Preliminary phytochemical screening of Aqueous & Petroleum ether extracts of “ Ziziphus Jujuba”.
2. To compare the changes in Quantification of infract size in normal and treated groups.
3.To compare the changes in SGOT levels in Control and treated groups.
REVIEW OF LITERATURE: Peng Wen Huaug, Hsieh Ming Tsuen et al.[11] have been reported the Hypnotic-Sedative and Anxiolytic effect of extract of Ziziphus jujube showed the significant anxiolytic effects.
S. Lee, B.Min, C. Lee et al. [12] have reporte the Anti-Cancer Activity. Fruit extracts of Z. jujube were tested against tumour cell lines.
Esteki Taranch and Urooj Asna et al. [13] have been reported the Antioxidant activity of Ziziphus jujuba Mill the results obtained in this investigation indicate that the peel is a rich source of many antioxidant compounds. The antioxidant activity of extracts was determined by DPPH and reducing power assay.
M.S. Ganachari, K. Shiv and K.G. Bhat et al. [14] have been reported the cardiovascular activity of leaf from Z. Mauritiana. These leaves were found to increase the endogenous prostaglandin 12 release (the most important natural inhibitor of the platelet aggregration has discovered a powerful vasodilator) from the rat aorta by upto 25.3% at 3 microg/ml.
Sampath Kumar, Arutla.R, Swaroopa.D Rao, K.S et al.[15] have reported the Wound healing activity of methanolic bark extract of Ziziphus jujuba showed the wound healing activity at the high dose (10% w/w) then the low dose (5% w/w) by exercise wound model for the period of 24 days in albino rats.
R.B. Gupta, S.Sharma, J.R.Sharma et al. have been reported the antifertility or contraceptive property of the ethyl acetate bark extract of Z. jujuba was found to have effect of antisteroidogenic activity and hence fertility in adult female mice. It was found to be arrest the normal estrus cycle of the adult female mice at the time of diestrus stage and reduced the wet weight of the ovaries significantly. Antifertility activities of crude extracts were found to be reversible in rat.
Robert W. Regenhardt has been reported understanding the cerebroprotective actions of the ACE2/ANG-(1-7)/MAS axis during ischemic and hemorrhagic stroke. These studies have increased the understanding of the mechanism by which activating the ACE2/Ang-(1-7)/Mas axis of the RAS is cerebroprotective in ischemic stroke.
M. Nandagopal P. Muralidharan et al. have reported the Root Extracts of Asparagus racemosus shows the cerebroprotective effect. In Global Cerebral Ischemia in Rats. The ischemia-induced brain injury of was protected by the oral administration of MEAR.
[adsense:468x15:2204050025]
 PLANT PROFILE:BOTANICAL INTRODUCTION:
PLANT PROFILE:BOTANICAL INTRODUCTION:
Name of the plant : ZIZIPHUS JUJUBA
Family : Rhamnaceae
Vernacular Names:
Marathi - Bor
Hindi - Ber
Bengali - Kul
Tamil - Ilanthai pazham
Kannada - Yelchi hannu
Telegu - Ragi pandu
EXTRACTION:The Powder was packed in a round bottom flask and extracted with aqueous at 100°C and with petroleum ether at 800C by Soxhlet extractor. [12] After extraction the residues was evaporated on water bath at required temperature to get a solid mass.
METHODOLOGY:
EXPERIMENTAL PROTOCOL: In the present study albino wistar rats weighing about 200-250gms were selected and divided into 5 groups containing 6 animals in each group.Rats were randomly divided into 5 groups each containing 6 animals.
Group 1: Control group.
Group 2: Animals treated with lead 0.1 ml/100 g body weight i.p
Group 3: Animals treated with Petroleum ether extract of Ziziphus Jujuba 250mg/kg
Group 4 : Animals treated with Petroleum ether extract of Ziziphus Jujuba 500mg/kg.
Note: All the groups were subjected to pre-treatment for a period of 7 days except Control group
DOSAGE: Based on the references of previous acute toxicity studies of the plant 250mg/kg & 500mg/kg doses were selected as low and high dose.
Induction of Cerebral Ischemia[1]: Cerebral ischemia was induced by temporary bilateral carotid artery occlusion followed by reperfusion. Rats were anaesthetised with thiopentone sodium[13] at a dose of 45mg/kg body weight and shifted to the surgery table. Check anaesthesia level intermittently (e.g., toe pinch) and maintain constant anaesthetic effect accordingly throughout the surgery. A small midline skin incision is made in neck. Rats were made ischemia by occluding two common Carotid Arteries with a silk thread for 10 minutes and reperfusion is allowed for 1hr by removing the thread. After 4hrs of reperfusion blood was withdrawn through carotid artery for biochemical estimations and brain is dissected to determine the percentage of cerebral infarction.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS:
PHYTOCHEMICAL TESTS:
| Tests | Aqueous extract | Petroleum ether extract |
| I. Tests for sterols | ||
| 1. Test solution+Conc. H2SO4 | - | - |
| 2. Salkowski’s test | - | - |
| 3. Liebermann Burchard’s test | - | - |
| II. Test for Glycosides | ||
| 1. Baljet’s test | - | - |
| 2. keller-killiani test | - | - |
| 3. Bromine water test | - | - |
| 4. Legal’s test | - | - |
| III. Test for Saponins | ||
| 1. Foam test | + | + |
| 2. Haemolysis Test | + | + |
| IV. Test for carbohydrates | ||
| 1. Molisch’s test | - | - |
| 2. Barfoed’s test | - | - |
| 3. Benedict’s test | - | - |
| V. Test for Alkaloids | ||
| 1. Mayer’s test | - | - |
| 2. Wagner’s test | - | - |
| 3. Dragondroff’s test | - | - |
| 4. Hager’s test | - | |
| VI. Test for Flavonoids | ||
| 1. Ferric chloride test | + | + |
| 2. Schinoda test | + | + |
| 3. Zn-HCL reduction test | + | + |
| 4. Alkaline reagent test | + | + |
| 5. Lead acetate test | + | + |
| VII. Test for Tannins | ||
| 1. Ferric chloride test | + | - |
| 2. Gelatin test | + | - |
| VIII. Test for Proteins | ||
| 1. Millon’s test | - | - |
| 2. Xanthoprotic test | - | - |
| 3. Ninhydrin test | - | - |
| IX. Test for Fixed oils and fats | ||
| 1. Spot test | - | - |
| X. Test for gums and Mucilages | ||
| 1.Swelling test | - | - |
| 2. Molisch’s test | - | - |
| XI. Test for Triterpenes | ||
| 1. Salkowski’s test | + | + |
| 2. Leibermann Burchard test | + | + |
SERUM PARAMETERS (SGPT)
Consolidated table showing Effect of AEZJ & PEEZJ on infarct size.
|
S.NO. |
Control |
AEZJ 250mg/kg |
AEZJ 500mg/kg |
PEEZJ 250mg/kg |
PEEZJ 500mg/kg |
|
1 |
1.27 |
0.91 |
0.48 |
1.08 |
0.63 |
|
2 |
1.35 |
0.89 |
0.39 |
1.02 |
0.59 |
|
3 |
1.20 |
0.94 |
0.42 |
1.12 |
0.62 |
|
4 |
1.42 |
0.85 |
0.46 |
1.06 |
0.69 |
|
5 |
1.32 |
0.87 |
0.31 |
0.98 |
0.55 |
|
6 |
1.22 |
0.81 |
0.46 |
1.04 |
0.69 |
|
Mean + SD |
1.29+ 0.08
|
0.87 + 0.04 |
0.42 + 0.06 *** |
1.05 + 0.06 |
0.62 + 0.05 *** |
Results are expressed as Mean ± S.D.
All groups are compared with control. n=6; p value is ˂ 0.0001***
All the values are denoted by mg/dl.
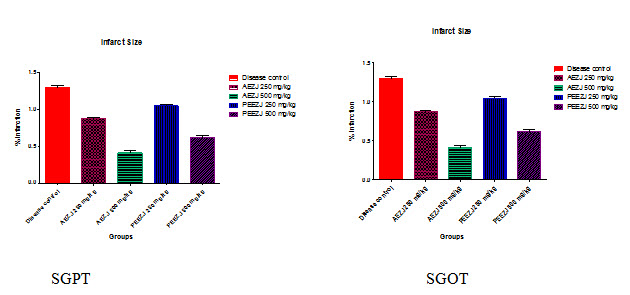
Consolidated table showing Effect of AEZJ & PEEZJ on SGOT:
|
S.NO. |
Control |
AEZJ 250mg/kg |
AEZJ 500mg/kg |
PEEZJ 250mg/kg |
PEEZJ 500mg/kg |
|
1 |
129 |
85 |
52 |
95 |
59 |
|
2 |
119 |
89 |
56 |
89 |
64 |
|
3 |
121 |
90 |
51 |
84 |
62 |
|
4 |
115 |
82 |
49 |
91 |
69 |
|
5 |
124 |
86 |
58 |
90 |
61 |
|
6 |
130 |
82 |
48 |
93 |
70 |
|
Mean + SD
|
123+ 5.83 |
85+ 3.38 |
52+ 3.93 *** |
90.33+ 3.77 |
64+ 4.44 *** |
Results are expressed as Mean ± S.D.
All groups are compared with control. n=6 ; p value is ˂ 0.0001***All the values are denoted.
QUANTIFICATION OF INFARCT SIZE:
At the end of 4 hours of reperfusion, animals were sacrificed with excess dose of anaesthesia. Brain was excised from skull rapidly and subsequently. The Brain was washed with normal saline, blotted dry and weighed. It was sliced to 0.1 cm thick sections and the slices were incubated in 1% TTC solution prepared in pH 7.4 phosphate buffer for 30 min. at 37°C. In viable cerebrum, TTC is converted by dehydrogenase enzymes [13] to a red formazan pigment that stains tissue to dark red. The infarcted cerebrum[14] that does not take TTC stain where the dehydrogenase enzymes are drained off remains pale in color. The pale necrotic cerebral tissue was separated from the stained portion [15] and weighed on an electronic balance. Cerebral infarct size was expressed quantitatively in terms of percent cerebral necrosis.
Histopathology
Rats treated with vehicle (G1) shows normal architecture of neuro glail cells and. Rats treated with lead(G2) shows extensive necrosis on neuronal cellswith enlarged glaicial cells. Rats treated with zizipus zuzuba (G3 and G4) and neuronal cells with enlarged glaicial cells shows normal architecture of and treated with 500 mg/kg dose shows better protection when compared with low dose (250 mg/kg). the results were presented in following figure.
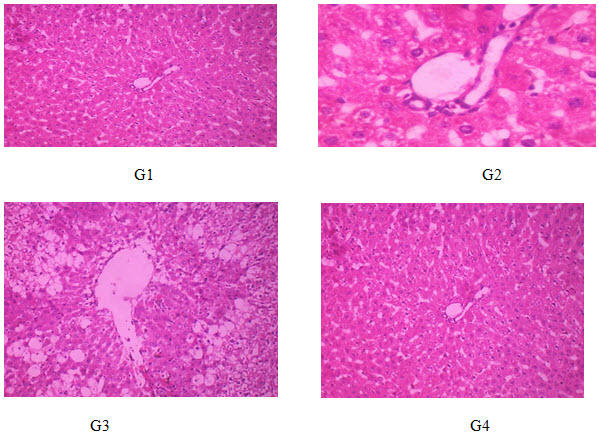
Histopathology of brain G1 – normal control rats were treated with vehicle. G2 – toxic control rats were treated with lead at a single dose 0.1 ml/100 g body weight i.p.G3 – rats were treated with Ziziphus Jujuba 250 mg/kg body weight p.o.G4 – rats were treated with Ziziphus Jujuba 500 mg/kg body weight p.o
DISCUSSION
Irreversible cell damage may occur during the prolonged stoppage of cerebral blood flow. Intervention such as thrombolysis, surgical endorctomy or angioplasty can restore blood flow to the affected tissue. However, restoration of blood flow following acute disruption is also accompanied by a further injurious phenomenon known as cerebral reperfusion- injury. A balance is maintained between oxidative attack and anti oxidant defence system in different tissues. Brain contains low level of antioxidative defence, it is increased level of oxidative stress seen in cerebral tissue. So the major contributing factor for the development of neuro-degenerative disease like cerebral ischemia reperfusion. When the oxygen free radicals are generated at the time of post ischemic reperfusion are the causative agents for the neuronal injury. So the elevation of conjugated diene levels, lipid peroxidation and cell damage was reported in the cerebral region after ischemia and reperfusion. Various epidemiological studies have evaluated an inverse correlation between flavonoids intake and the risk of death causes from cerebral injury. Many flavonoids showed that improved functional recovery in the rat brain during ischemic and reperfusion
Experimental studies reveal that aqueous extract & petroleum ether extract from Ziziphus Jujuba (250mg/500mg/kg) orally administered for 7days produce a significant decrease in the infract size level and decrease in the SGOT levels in the model of cerebral infraction reperfusion-injury in albino wistar rats.
CONCLUSION
In the present investigation the protective effect of drug Ziziphus Jujuba was evaluated in experimentally induced cerebral ischemia reperfusion injury in rat model pre-treatment with the Ziziphus Jujuba leaf extracts. The protective effect of the aqueous extract of Ziziphus Jujuba may be due to the presence of flavonoids, saponins, triterpenoids and tannins. In petroleum ether extract of Ziziphus Jujuba the protective effect may be due the presence of flavonoids, saponins and triterpenoids. There was a dose dependent increase in cerebral protection in terms of reduction of infarct size of brain tissue and SGOT levels in serum. Furthermore investigation is needed to find out the particular constituent which is responsible this protective activity.
REFERENCES
1. Neelima. H, Annapurna akula. G,Wade. S; Evaluate the cerebro protective activity of querceti rutin in ischemia reperfusion induced cerebral infarction in rats; Pharmacology online; 2009; 68(3); 216-229
2. Smith WS; Pathophysiology of focal cerebral ischemia:a therapeutic perspective; J Vasc Interv Radiol; 2004; 15(1 pt 2); s3-s12.
4. Muge kiray J, Husnu alper K. Neelima I; Protective effects of deprenyl in transient cerebral ischemia in rats; Chinese journal of physiology; 2008; 51(5); 275-281.
5. Izumi Harukuni.P, Anish Bhardwaj.N; Mechanisms of brain injury after global cerebral ischemia; neurol; clin; 2006; 54(7); 1-21.
6. Ashu Aggarwal.G, Mamata khatak.F; Cerebral ischemic stroke: sequels of cascade; international journal of pharma and biosciences; 2010; 1(3); 1-24
7. Aluclu MU, Arslan S, Acar A, Guzel A, Bahceci S, Yaldiz M.; Evaluation of effects of memantine on cerebral ischemia in rats; neurosciences; 2008; 13(2); 113-116.
8. SV Levich, KV Alesksandrova et al; Cerebroprotective activity of 3-benzylxanthine derivative-compound Ale-15, in conditions of bilateral common carotid arteries ligation (ischemic stroke); Int J Basic Clin Pharmacol.; 2013; 2(6); 705-710
9. S Arumugam, S Kavimani, B Kadalmani, ABA Ahmed, MA Akbarsha, MV Rao; Anti-diabetic activity of leaf and callus extracts of Aegle marmelos in rat; 2008; 34; 317-321.
10. Satoskar RS, Bhandarkar SD, Ainapure SS; Pharmacology and Pharmacotherapeutics; 5th ed; Mumbai; popular prakshan; 1992; 863-865.
11. Peng Wen D., Huang, Hsieh N., MingTsuen M; Hypnotic-sedative and Anxiolytic effect of extract of Ziziphus jujuba showed the significant anxiolytic effects; Ethano pharmacology; 2000; 72(3); 435-441.
12. Lee SM, Min BS, Lee CG, Kim KS, Kho YH; cyto-toxic triterpenoids from the Fruit extract of Zizipus Jujuba; Planta Medica; 2003; 69(11); 1051-1054.
13. Esteki Taraneh C., and Urooj Asna D.; Antioxidant Activity of Ziziphus jujuba Mill; Journal of pharmacy research; 2012;5(5); 2075-2079.
14. Ganachari M.S., Shiv K.,and Bhatt K.G. et al.; Effect of Ziziphu jujuba leaf extract on phagocytosis by Human neutrophils; Journal of Natural remedies; 2004; 41(2); 47-51.
15. Sampath Kumar CH., Arutla.R, Swaroopa.D, Rao K.S et al; Wound healing potential of Ziziphus jujuba bark extract on albino rats; International journal of research in ayuverda and pharmacy; 2012; 3(6); 830-832
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









