{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Deepa Vishwakarma, Deepa Dhiman, Amit kumar Pal, Aman Mittal, Sunit Saini
Department of Pharmacy,
Smt. Tarawati Institute of Biomedical Allied Sciences,
Roorkee, Uttarakhand, India
vishwakarmadeepa152@gmail.com
ABSTRACT
The objective of our investigation was to design a thermodynamically stable and dilutable nanoemulsion formulation of Ibuprofen, with minimum surfactant concentration that could improve its solubility, formulation were taken from thermodynamic stability and dispersibility test. The oil, surfactant, co-surfactant and aqueous phase, respectively, containing 100mg of ibuprofen showed a significant improvement in drug release. In vitro drug release of the nanoemulsion formulation was highly significant as compared to marketed tablet formulation. The present study revealed that tinidazole nanoemulsion could be used as a liquid formulation for increase bioavailability. The formulated system can also be treated as self nano emulsifying drug delivery system.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2455
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 1 Received On: 16/08/2016; Accepted On: 03/09/2016; Published On: 01/01/2017 How to cite this article: Dhiman D, Pal AK, Mittal A, Saini S; Preparation and evaluation of nano-emulsion formulation by using spontaneous emulsification; PharmaTutor; 2017; 5(1); 54-58 |
INTRODUCTION
The term “Nanoemulsion” refers to a thermodynamically stable isotropic clear dispersion of two immiscible liquid such as oil and water, stabilized by an interfacial film of surfactant molecules. A nanoemulsion is considered to be a thermodynamically or kinetically stable liquid dispersion of an oil phase and a water phase in combination with a surfactant. The dispersion phase typically comprises small particles or droplets, with a size range of 5nm-200nm, and has very low oil/water interfacial tension. Because the droplet size is less than 25% of the wavelength is formed readily and sometime spontaneously generally without high energy input. In many cases a co-surfactant or co-solvent is used in addition to the surfactant, the oil phase and the water phase.
There types of nanoemulsion are most likely to be formed depending on the composition.
- Oil in water nanoemulsion where in oil droplets are dispersed in the continuous oil phase.
- Water in oil nanoemulsion where in water droplets are dispersed in the continuous oil phase.
- Bi-continuous nanoemulsion where in micro domains of oil and water are interdispersed with in the system.[1,2,3]
Advantages of nanoemulsion over other dosage forms[4]
- Increase the rate of absorption.
- Various routes like topical oral and intravenous can be used to deliver the product.
- Increase bioavailability.
- Help in taste masking.
- Rapid and efficient penetration of the drug moiety
Components of nanoemulsion[5]
Main three components of nanoemulsion are as follows:
1. Oil
2. Surfactant/ co-Surfactant
3. Aqueous phase
Application of nanoemulsion
- Parenteral delivery
- Oral drug delivery
- Topical drug delivery
- Ocular and pulmonary delivery
- Nanoemulsion in Biotechnology
MATERIALS
Ibuprofen was received as a gift sample from Aglowmed drugs Pvt Ltd; isopropyl myristate, tween 80, span80 was procured from central drug house; and ethanol was obtained from chaugshu yangyuan chemical (china).
METHODS
Preparation of standard curve
The absorption maxima of drug were determine by UV-Spectrophotometer and was used for preparation of standard curve in pH 7.2 phosphate buffer solution. Various concentrations of 2,4,6,8, & 10 microgram per milliliter sample were prepared and analyzed for absorbance. The data was then treated statistically to obtain the value of Y= MX+C.
Selection of Phases
The entire water insoluble drug was tasted for solubility in isopropyl myristate in presence of surfactant co- surfactant mixture. The drug with best solubility profile in oily phase was investigated.
Preparation of semi-permeable membrane
0.1 N Hcl was prepared by dissolving 43.47 ml Hcl and made up the volume up to 500 ml by distilled water. After that the egg was dipped in the solution carefully, until the outer membrane of the egg was removed. After complete removal the prepared semi permeable membrane than it was washed with distilled water to remove the acid solution completely
Preparation of nanoemulsion
The homogeneous organic solution (S1) was composed of oil (400mg isopropyl myristate) and a lipophilic surfactant (86 mg of span 80) in water miscible solvent (40ml). The homogeneous aqueous phase (S2) was formed by water (80ml), and hydrophilic surfactant (136mg Tween 80). - The organic phase was injected in the aqueous phase under magnetic stirring. The o/w emulsion was formed instantaneously by diffusion of the organic solvent in the external aqueous phase leading to the formation of nano droplets. The magnetic stirring was maintained during 30 min to let the system reach equilibrium. - The totality of the water miscible solvent was removed by evaporation during 45 min under reduces pressure. Nano droplets of oil were dispersed in aqueous solution of water and hydrophilic surfactant. [6,7,8,9,10,11]
Pseudo-ternary phase diagram study
On the basis of the solubility studies of drug isopropyl myristate was selected as the oil phase. Tween 20 and ethanol were used as surfactants and co- surfactant respectively. Water was used as an aqueous phase for the construction of phase diagram. Oil surfactants and co-surfactant (Smix) were grouped together for phase study. Surfactant and co-surfactant in group was mixed in weight ratio (1:1). For phase diagram oil and specific ratio was mixed thoroughly in different weight ratios from 1:9 to 9:1 in different glass vials. Sixteen different combinations of oil and Smix, 1:9, 1:8 1:7, 1:6, 1:5, 2:8 (1:4), 1:3.5, 1:3, 3:7(1:2.3), 1:2, 4:6(1:1.5), 5:5(1:1), 6:4(1:0.7), 7:3(1:0.43), 8:2(1:0.25), 9:1(1:0.1) were made so that maximum ratio were covered for the study to delineate the boundaries of phases precisely formed in the phase diagrams. Pseudo-ternary phase diagram were develop using aqueous titration method. Slow titration with aqueous phase was done to each weight ratio of oil and Smix and visual observation was carried out for transparent and easily flow able oil in water nano emulsion. The physical state of nano emulsion was marked on a pseudo three component phase diagram with one axis representing aqueous phase. The other representing oil and the third representing a mixture of surfactants and co-surfactants at fixed weight.
EVALUATION PARAMETERS
Dispersibility Test
The efficiency of self emulsification of oral nanoemulsion was assessed using a standard USP XXII dissolution apparatus 2.1 ml of each formulation was added to 500ml of water at 37oC. A standard stainless steel dissolution paddle rotating at 50 rpm provided gentle agitation. The in vitro performance of the formulation was visually assessed using the following grading system.
Grade A: rapidly forming (with in 1 min) nanoemulsion having a clear or bluish appearance.
Grade B: rapidly forming, slightly less clear emulsion having a bluish white appearance.
Grade C: fine milky emulsion that formed with in 2 min.
Grade D: dull greyish white emulsion having slightly oily appearance that is slow to emulsify (longer than 2min)
Grade E: formulation exhibiting either poor or minimal emulsification with large oil globules present on the surface.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Thermodynamic stability study
1. Heating cooling cycle: six cycle between refrigerator temperature 40°C and 45°C with storage at each temperature of not less than 48 hrs was studied. Those formulations which were stable at this temperature were subjected to centrifugation test.
2. Centrifugation: passed formulation were centrifuged at 3500rpm for 30 min. those formulations that didn’t show any phase separation were taken for the freeze thaw stress test.
3. Freeze thaw cycle: three freeze thaw cycles between 21°C and 25°C with storage at each temperature for not less than 48hrs was done for the formulation. Those formulation which passed these thermodynamic stress test, were further taken for the dispersibility test for assessing the efficiency of self emulsification.
In-Vitro drug delivery
In-vitro release test was performed in 500ml of distilled water which was based on USP XXIV method (dissolution apparatus #2, at 50rpm and 37°C). 5.2 ml of Nano emulsion formulation (single dose containing 100mg of ibuprofen) was placed in dialysis bag. Samples 1ml were withdrawn at regular time intervals (0, 10, 20, 30, 40, 50, 60 minutes) and desire amount of dissolution media was replaced. The release of drug from nanoemulsion formulation was compared with the conventional tablet formulation. The samples were analyzed for the drug content using UV method at 221 nm. In-vitro drug release data were analyzed by one way analysis of variance.[5, 12]
RESULT AND DISCUSSION
The absorption maxima of drug as determined by UV- Spectrophotometer was found to be 221nm for Ibuprofen in 7.2 pH phosphate buffer solution, when scanned between the range 200-400 nm. The method of analysis using UV Spectrophotometer of at 221 nm respectively was found to be reproducible. Standard calibration curve of Ibuprofen was prepared in 7.2 pH buffer at the wavelength 221nm respectively using UV-Spectrophotometer. The absorption data of Ibuprofen presented in table were found to obey Beer’s law within the specified range as indicated by statistical analysis under taken.
|
S. No. |
Concentration(mcg/ml) |
Absorbance (nm) |
|---|---|---|
|
1 |
0 |
0 |
|
2 |
2 |
0.0464 |
|
3 |
4 |
0.1682 |
|
4 |
6 |
0.2102 |
|
5 |
8 |
0.3042 |
|
6 |
10 |
0.4451 |
Table 1: calibration curve data for Ibuprofen in 7.2 pH Phosphate buffer at 221 nm

Fig. 1: calibration curve of Ibuprofen in 7.2 pH Phosphate buffer at 221 nm
Selection of phases
Ibuprofen was a poorly water soluble drug. So that oil (isopropyl myristate) was preferred for better solubility of Ibuprofen.
Preparation of Nanoemulsion
It was prepared by mixing with the isopropyl myristate to form oily phase, it was enriched by the addition of surfactant and co-surfactant mixture, finally nanoemulsion was formed.
Pseudo- ternary phase diagram study
Tween 80 and ethanol were used as surfactant and co- surfactant respectively. Water was used as an aqueous phase for the construction of phase diagram. Constructing phase diagram is time consuming particularly when the aim is to accurately delineate a phase boundary. When Ibuprofen was used alone without co-surfactant, very low amount of oil (1ml) could be solubilized at a high concentration of surfactant. As the construction of surfactant was increased solubilization of oil decreased when co-surfactants was added with surfactant in equal amount (Smix ratio1:1). It was seen that nanoemulsion area decreased as compared to 1:1 ratio and here also only up to 1ml oil could be solubilized with surfactant concentration 1 part of co-surfactants the nanoemulsion area decreased further and maximum amount of oil that could be solubilized was 15% w/w and that too at higher concentration of Smix. It can be observed that the formulation prepared from phase diagram in which the nanoemulsion area was extended towards aqueous rich apex could be diluted to a larger extent. As co-surfactant concentration was increased from 1:1 to 1:3 to the nanoemulsion region reduced
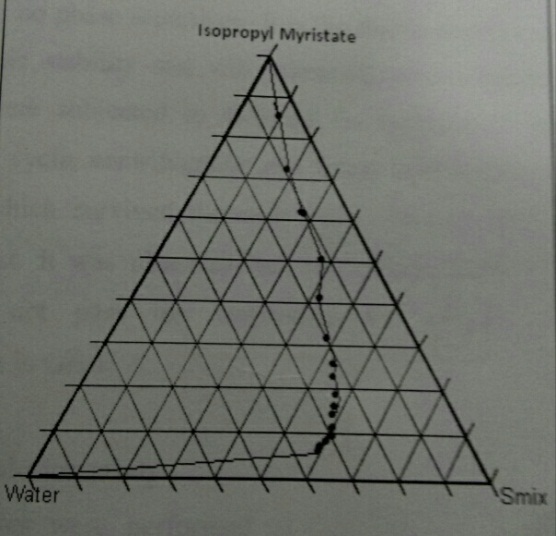
Fig 2: Pseudo- ternary phase diagram
Dispersibility test
When infinite dilution is done to nanoemulsion formulation there is every possibility of it to phase separate, leading to precipitation of poorly soluble drug as nanoemulsion are formed at a particular concentration of oil, surfactant co-surfactant and water. For oral nanoemulsion the process of dilution by the GI fluids will result in the gradual desorption of surfactant located at the globule interface. The process is thermodynamically driven by the requirement of the surfactant to maintain an aqueous phase concentration. We used distilled water as a dispersion medium because it was well reported that there is o significant difference in the nanoemulsion prepared using surfactant, dispersed in either water or simulated gastric or intestinal fluid.
Thermodynamic stability studies
Nanoemulsion is thermodynamically stable system and is formed at a particular concentration of oil, surfactant, co-surfactant and water with no phase separation. It is the thermodynamically are nanoemulsion that gave kinetic stability and will eventually phase separate. The selected formulations were subjected to different thermodynamic stability by using heating cooling cycle, centrifugation and freeze that cycle stress tests. Those formulations, which survived thermodynamic stability tests, were taken for dispersibility test. It was observed that formulation prepared from Isopropyl myristate did not pass the thermodynamic stability tests that passed dispersibility test in Grade A.
In vitro dissolution study
Dissolution studies were performed to compare the release of drug from Ibuprofen nanoemulsion formulation and conventional tablet formulation, having the same quantity (100 mg) of Ibuprofen. The release of drug from nanoemulsion formulations was highly significant. When compared to marketed tablet, higher release was obtained in case of nanoemulsion. As compared to tablet formulation which showed slower release in one hour nanoemulsion showed a significantly higher release. This is because of small globule size, and eventually higher surface are in case of nanoemulsion, which permit faster rate of drug release.
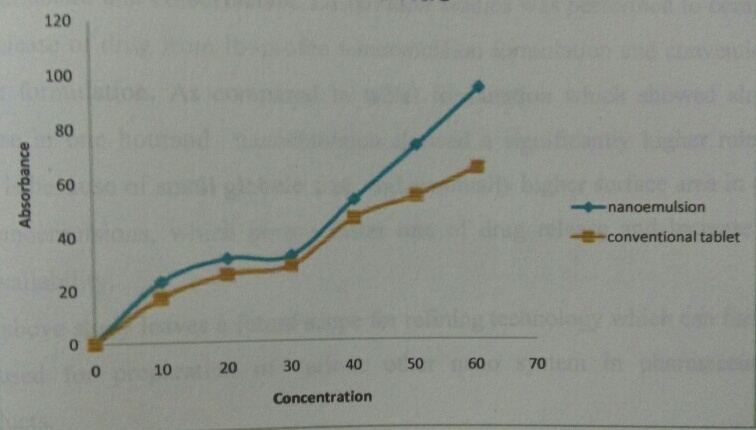
Fig. 3: Release profile of nanoemulsion formulation and tablet at 7.2 pH phosphate buffer solution
CONCLUSION
In this study, we got success in the development and evaluation of nanoemulsion. Based on higher drug release and lower surfactants concentration, higher solubility as well as higher bioavailability without variable absorption has been optimized as nanoemulsion formulation of ibuprofen as oil, surfactants and co-surfactants. Dissolution study was performed to compare the release of drug from ibuprofen nanoemulsion formulation and conventional tablet formulation. As compare to tablet formulation which showed slower release in one hour and nanoemulsion showed a significantly higher release. This is because of small globule size and eventually higher surface area in case of nanoemulsion, which permit faster rate of drug release and increase the bioavailability. The above study leaves a future scope for refining technology which can further be used for preparation of various other nano systems in pharmaceutical products
REFERENCES
1. Shinoda K, Lindman B; Organised surfactant system : Emulsion; Langmuir 1987; 3(2); 135-149.
2. Tenjrala S N; Microemulsion An Overview and pharmaceutical application; Critical Reviews TM in therapeutic Drug carrier system; 1999; 16(5); 461-521
3. Ktistis G, Niopas I; A study on the in-vitro percutaneous absorption of propranolol from disperse system; J. Pharm Paracetamol; 1998; 50(4); 413-418.
4. Alvarez- Figueora M J , Blanco Mendez J; Transdermal delivery of methotrexate Iontophoretic delivery from hydrogels and passive delivery from microemulsion; Int J pharm; 2001; 215(1-2); 57-65
5. Kim Y H, Ghanem A H, Mahmoud H, Higuchi W I; Short chain alkanols as transport enhancers for lipophilic and polar ionic permeants in hairless mouse skin mechanism of action; Int.J Pharm; 1992;80(1-3); 17-31.
6. Binks B. P et al; Interfacial tension minima in oil- water- asurfactant system J. Chem. Soc., Faraday Trans. 1; 1986; 82(1);125-142.
7. Becher, D.Z., application in agriculture; In: Becher, P. (Ed.), encyclopedia of emulsion technology; first edition, Marcel dekker, New York; 1983; p-239-320.
8. Bouchemal K., Briancon S., Perrier E., Fessi H., bonnet I., Zydowicz N. ; Synthesis and characterization of polyurethane and poly (ether urethane) nanocapsules using a technique of interfacial polycondensation combined to spontaneous emulsification; Int. j. pharm; 2004; 269 (1); 89-100.
9. Bru Guillon X Breton P Couvreur, P, Lescure, F, Roques carmes C, Riess, G, Procedes de- prepation de-nanoparticals contenant eventuellement uneosplusieurs molecules biologiquement active et compositions pharmaceutiques les contenant; 1998.
10. Davies J T, Haydon D, A Spontaneous emulsification. Int. Congr. Surf Act; 1957; 2nd ,417-425. Davies Rideal E K,; Diffusion through interfaces. In Willmer ,H; interfacial Phenomena first academi press, new york;1961; pp.343-450.
11. A Book ; Nakajima H,; In solans C, Konieda H, Industrial Application of microemulsion; Marcel Dekker, New york;1997; A novel method to correlate emulsion viscosity data; Colloid surf; 1998; 275-286
12. Mao L, Yacan Y, Gao Y et.al.; characterization and stability evaluation of B-carotene nanoemulsions prepared by high pressure honoginization under various emulsifying conditions food; res. Ins-2008; 41(1):61-68
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









