About Authors:
*Parasiya Sachin kumar R., Balamuralidhara V., Pramod Kumar T.M., Rohit Gujarati, Abhisek Shukla, Vandana Kshatri
Pharmaceutical Regulatory Affairs Group, Dept. of Pharmaceutics,
JSS College of Pharmacy, JSS University, S.S Nagar, Mysore-570015, Karnataka, India
*sachin_patel2007@yahoo.com
{ DOWNLOAD AS PDF }
Abstract
Across Asia, a convergence of economic trends, government policies and greater awareness among the general public of healthcare issues has created an environment that is poised for dramatic growth and change. Taiwan, for instance, can be taken as an example. Taiwan has one of Asia's most highly-praised healthcare systems with excellent provision of healthcare and key health outcomes. Nevertheless, the government is facing new pressures for public healthcare reforms as result of a rapidly ageing population and rising healthcare costs. This paper provides an introductory overview of Taiwan’s sudden changes in its drug regulations due to TFDA (The Taiwan Food and Drug Administration) establishment in 2010, TFDA of the Department of Health (DOH) made an advance announcement about the “amendment draft of the Provisions Governing the Registration and Market Approval of Drugs”, which amends a total of 40 Articles. Without impeding the quality, safety and therapeutic effect of drugs, most of the amended Articles are about simplification of application procedures and loosening of regulations for drug registration and market approval. Regulations loosened are imposed on new drugs, radioactive drugs, allergenic drugs and drugs for export that is intended to accelerate the process to sell new drugs on the market and promote the export of domestically manufactured drugs. As a result of these changes in regulations many pharmaceutical MNCs and local manufacturers explored their business in Taiwan due to quick approval of their NDAs and gained more flexibility in the local market. To support Taiwan's generic drug industry, DOH has also decided to take measures to simplify and reduce the ANDA application time.
REFERENCE ID: PHARMATUTOR-ART-2098
Introduction:
Taiwan is considered as one of the best market to explore the pharmaceutical drug products. It is the sixth largest pharmaceutical market in the Asia Pacific region1. Newer approach has been updated for registration of foreign drugs in Taiwan market.
Roles of Regulatory Authorities:
* Public Health Protection
· Gate-keeper
- Prudent evaluation based on Good Review Practice
- Drug quality, safety and efficacy
* Health Industry Promoter
· Consultation mechanism
· Efficient and transparent review process
· International harmonization
Pharmaceutical Regulation in Taiwan:
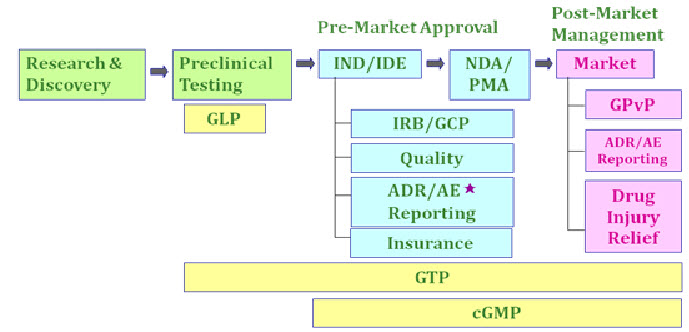
Taiwan market status in the past:
As per old provisions, registration of chemical entity was not possible without a certificate of pharmaceutical product (CPP). This was the main drawback behind Taiwan’s participation in the R&D and the clinical Trials with rest of the world and thus a long time was needed for the approval of the new investigational drug.
Taiwan market status present:
The new provision permits registration of new chemical entity without a certificate of pharmaceutical product (CPP), as long as the relevant clinical trials have been carried out and a relevant regulation has been provided.This help for the quick registration on new drug in Taiwan.
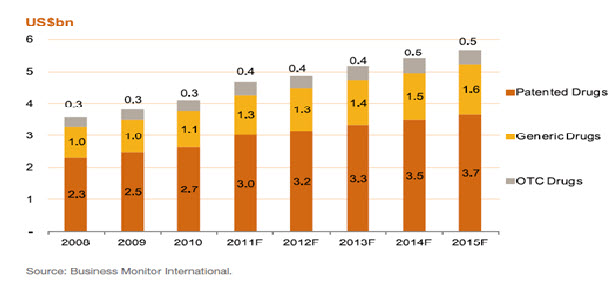
Taiwan’s pharma industry2 was worth around US$4.7bn in 2011, having grown by an annual average of 9.2% between 2008 and 2011. Business Monitor International projects growth will slow to 5.1% p.a. in 2011-2015 due to downward price pressure. High volume consumption of pharmaceuticals per capita continues to drive the market, but the government is keen to control public healthcare costs. One of its strategies is to encourage a shift towards consumption of generics at the expense of originator drugs. Therefore, these drugs are forecast to continue to take share from branded medications.
Main changes in the regulations:
Application for the registration of new drugs with new ingredients without submitting a CCP must meet the following conditions3:
- Phase I and Phase III trials must be carried out in Taiwan simultaneously with foreign institutions, or Phase II and Phase III pivotal trials must be carried out in Taiwan simultaneously with overseas institutions; in addition, the size of the trial group in Taiwan must meet specific requirements, and the results of Phase III pivotal trials must be similar to those of the overseas trials.
- The results of the clinical trials must have been inspected and approved by the domestic health authority (TFDA).
- Proof that trials comply with the requirements of Taiwan’s Medical Treatment Act and with Good Clinical Practice (GCP) must be provided if requested by the competent authority.
- At least 10 persons must be involved in Phase I clinical trials conducted in Taiwan, minimum 20 persons must be involved in Phase II clinical trials, and at least 80 persons must be involved in Phase III trials.
- The Taiwan Food and Drug Administration (TFDA) of the Department of Health (DOH) made an advance announcement about the “amendment draft of the Provisions Governing the Registration and Market Approval of Drugs”, which amends a total of 40 Articles.
Key points of the amendment include4:
- Simplified application procedures with substitutive regulations; a total of 18 Articles and 8 Appendices were amended.
- To stay in track with international drug control trend and practical needs, a total of 9 Articles were amended to stress quality control by adding definition of bio similar drugs and required technical documents for registration and market approval of this type of drugs, and regulations stipulating to specify sources of main active ingredients.
- In coordination to actual practical circumstances and consolidation of governmental institutions, and to allow consistency throughout the entire text, relevant regulations and words of 13 Articles were amended.
Impact on global pharma market:
The major impact of CCP and the amendments of the article encourage more number of pharmaceutical industries (domestic and international) for new drug registration as the consumption of time for registration is decreased. This resulted in rapid increase of Taiwan’s share in world pharma market as more MNCs started establishing business due to loosened drug registration procedures in Taiwan.
Milestones on Drug Regulation:
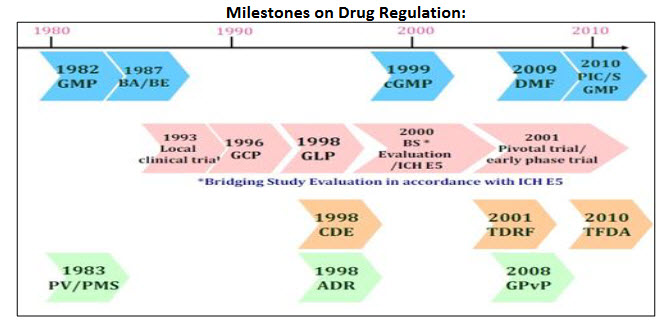
- 1982 Good Manufacturing Practice (GMP)
- 1987 Bioavailability / Bioequivalence Guideline (BA/BE)
- 1993 Safety monitoring system-the requirement of local clinical trial
- 1996 Good Clinical Practice (GCP)
- 1998 Good Laboratory Practices (GLP)
- 1999 Establishment of Adverse Drug Reaction reporting system
- 1999 Amendment of current GMP (cGMP)
- 2004 Implementation of bridging study evaluation (BSE)
- 2005 Revision of Pharmaceutical Affairs Act (Data Exclusivity & compulsory requirement of serious ADR reporting)
- 2008 Good Pharmacovigilance Practice (GPvP)
- 2008 Guideline for Biosimilar Product Review and Approval
- 2009 Drug Master File (DMF)
- 2010 Implementation of PIC/S GMP
- 2010 Amendment of Guideline for Biosimilar Product Review and Approval (in process)
- 2010 Develop strategies for review guidelines of tNCE and Botanical New Drug (in process)
- 2010 Relaxation of CPP requirement (in progress)
Conclusion:
The present paper focuses on the regulations that govern the registration of the new drugs, radioactive drugs, allergenic drugs, the guidelines applicable, the timeline issues applicable for the registration of the medicinal products, and the cost involved in registering new and generic drug products. The new regulations improveconsultation mechanism to facilitate industrial development and also increase international competitiveness.
References:
1. pacificbridgemedical.com May 13, 2010 /PRNewswire/ -- Pacific Bridge Medical.
2. pwc.tw/en_TW/tw/industries/publications/assets/healthcare-en.pdf
3. cepd.gov.tw Taiwan New Economy Newsletter No.105, issue date?2009-10-12.
4. fda.gov.tw
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 25/012/2014; Accepted On: 01/01/2014; Published On: 15/01/2014 How to cite this article: SKR Parasiya, Balamuralidhara V., Pramod Kumar T.M., R Gujarati, A Shukla, V Kshatri, A Paradigm Shift in Drug Regulations in Taiwan, PharmaTutor, 2014, 2(1), 162-165 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









