{ DOWNLOAD AS PDF }
 ABOUT AUHTORS
ABOUT AUHTORS
Hnawate R.M*., Deore P.
Dr. Vedprakash Patil Pharmacy College,
Aurangabad,
Maharashtra, India
ravi_hanwate@yahoo.co.in
ABSTRACT
For the past few years, there has been a considerable research on the basis of Novel drug delivery system, using particulate vesicle systems as such drug carriers for small and large molecules. Nanoparticles, Liposomes, Microspheres, Niosomes, Pronisomes, Ethosomes, Proliposomes have been used as drug carrier in vesicle drug delivery system. Nanotechnology refers to the creation and utilization of materials whose constituents exist at the nanoscale; and, by convention, be up to 100 nm in size.. Nanoparticles are being used for diverse purposes, from medical treatments, using in various branches of industry production such as solar and oxide fuel batteries for energy storage, to wide incorporation into diverse materials of everyday use such as cosmetics or clothes, optical devices, catalytic, bactericidal, electronic, sensor technology, biological labelling and treatment of some cancers. Various polymers have been used in the formation of Nanoparticles. Nanoparticles have been improving the therapeutic effect of drugs and minimize the side effects. Basically, Nanoparticles have been prepared by using various techniques as such dispersion of preformed polymers, polymerization of monomers and ionic gelation or co-acervation of hydrophilic polymer. Nanoparticles have been evaluated by using parameters of drug entrapment efficiency, particle shape, drug release study
[adsense:336x280:8701650588]
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 5 Received On: 24/01/2017; Accepted On: 08/02/2017; Published On: 01/05/2017 How to cite this article: Hnawate RM, Deore P;Nanoparticle - novel drug delivery system: A Review; PharmaTutor; 2017; 5(5);9-23 |
REFERENCE ID: PHARMATUTOR-ART-2490
INTRODUCTON:
In the novel drug delivery systems (NDDS), there are various novel carriers which have advantage over conventional dosage forms. Conventional dosage forms show high dose and low availability, in-stability, first pass effect, plasma drug level fluctuations and rapid release of the drug.2 NDDS is one of the important tool expanding drug markets in pharmaceutical industry. NDDS can minimize problems by enhancing efficacy, safety, patient compliance and product shelf life.3
Nanoparticles are of current interest because of an emerging understanding of their possible effects on human health and environmental sustainability, and owing to the increasing output of man-made nanoparticles into the environment. Nanoparticles are used in many different applications and created by many different processes. Their measurement and characterization pose interesting analytical challenges.
Particles having diameter in range between 10-100 nm are known as Nanoparticles. They are used as targeted delivery system for delivery of small and large molecules by changing their pharmacodynamics and pharmacokinetic properties. They can be defined as system which contain active ingredient dissolved, encapsulated or adsorbed in matrix material which are used as target delivery system.To see the effect of drug in target tissue, to increase stability against degradation through enzymes and for solubilization at intra-vascular route nanoparticles have been used. During the designing of nanoparticle some control has to take in care such as their release pattern, their size and surface properties which determine site-specific action at optimal rate with right dose regimen.Nanoparticles are sub-nano sized colloidal structure of synthetic or semi synthetic polymer. The first reported nanoparticles were based on non-biodegradable polymeric system (polyacrylamide, polymethyl-methaacrylate, polystyrene). The polymeric nanoparticles can carry drug(s) or proteineous substances, i.e. antigen(s). These bioactives are entrapped in polymer matrix as particulates or solid solution or may bound to particle surface by physical adsorption or chemically. The drug(s) may be added during preparation of nanoparticle or to the previously prepared nanoparticles. The term particulates aresuggestively general and doesn’t account for morphological and structural organization of system. Nano medicine isan emerging field of medicine with novel applications1.
Definition: Nanoparticles are defined as particulate dispersions or solid particles with a size in the range of 10-1000nm.The drug dissolved, entrapped, encapsulated or attached to nanoparticles matrix. Nanoparticles (including nanospheres and nanocapsules of size 10-200 nm) are in the solid state and are either amorphous or crystalline4-7. Polymeric materials have been extensively used for the preparation of nanoparticles8. Depending upon the method of preparation, nanoparticles, nanospheres or nanocapsules can be obtained. Nanocapsules are systems in which the drug is confined to a cavity surrounded by a unique polymer membrane, while nanospheres are matrix systems in which the drug is physically and uniformly dispersed.
In recent years, biodegradable polymeric nanoparticles, particularly those coated with hydrophilic polymer such as poly (ethylene glycol) (PEG) known as long-circulating particles, have been used as potential drug delivery devices because of their ability to circulate for a prolonged period time target a particular organ, as carrier of DNA in gene therapy, and their ability to deliver proteins, peptides and genes1-4.
The formulation of nanoparticles as targeted drug delivery system has been extensively studied 9. Targeted drug delivery can be achieved either by active targeting or passive targeting.
Active targeting of drugs can be attained either by conjugating drug molecule with tissue specific or cell specific ligand10 .Whereas, passive targeting of drugs can be attained either by incorporating drug molecule into a microparticles or nanoparticles.
Nanoparticles (NP) are colloidal drug delivery system which are formulated by natural, synthetic, and semi synthetic polymers. Particle size of NP ranges from 10 nm to 1,000 nm in diameter11. This colloidal drug delivery system shows different inner structure.
• Nanospheres in matrix type system
• Nanocapsules in reservoir type system
Need For Study12
At present 95% of all new potential therapeutics have poor pharmacokinetic and biopharmaceutical properties. Therefore, there is a need to develop suitable drug delivery systems that distribute the therapeutically active drug molecule only to the site of action, without affecting healthy organs and tissues, also lowering doses required for efficacy as well as increasing the therapeutics indices and safety profiles of new therapeutics. Different reasons are,
1) Pharmaceutical
- Drug instability in conventional dosage form
- Solubility
2) Biopharmaceutical
- Low absorption
- High membrane bounding
- Biological instability
3) Pharmacokinetic/ Pharmacodynamic
- Short half life
- Large volume of distribution
- Low specificity
4) Clinical
- Low therapeutic index
Objective12
The major goals in designing nanoparticles as a delivery system are to control particle size, surface properties and release of pharmacologically active agents in order to achieve the site-specific action of the drug at the therapeutically optimal rate and dose regimen
To achieve a desired pharmacological response at a selected site without undesirable interactions at other site, thereby the drug have a specific action with minimum side effects & better therapeutic index.
Ex: in cancer chemotherapy & Enzyme replacement therapy.
Ideal Characteristics12
Targeted drug delivery system should be
Biochemically inert (non-toxic), non-immunogenic
Both physically & chemically stable in vivo & in vitro.
Restrict drug distribution to target cells (or) tissues (or) organs & should have uniform capillary distribution. Controllable & predicate rate of drug release. Drug release does not affect drug action. Therapeutic amount of drug release. Minimal drug leakage during transit.
Carriers used must be biodegradable (or) readily eliminated from the body without any problem & no carrier induced modulation of diseased state. The preparation of the delivery system should be easy (or) reasonably simple reproductive & cost effective.
Advantages and Disadvantages13
Advantages of nanoparticles:
1. They are biodegradable, non- toxic, site specific and capable of being stored for at least one year.
2. They are capable of targeting a drug to a specific site in the body by attaching targeted ligands to surface of particles or use of magnetic guidance.
3. They offer controlled rate of drug release and particle degradation characteristics that can be readily modulated by the choice of matrix constituents.
4. Drug loading is high and drugs can be incorporated into the systems without any chemical reaction; this is an important factor for preserving the drug activity.
5. They offer better therapeutic effectiveness and overall pharmacological response/unit dose.
6. The system can be used for various routes of administration including oral, nasal, parentral, intra-ocular etc.
7. Particle size and surface characteristics of nanoparticles can be easily manipulated to achieve both passive and active drug targeting after parenteral administration.
Disadvantages
1. Presents bioacceptibility restrictions.
2. Difficult to manufacture in large scale.
3. Due to their small particle size and large surface area can lead to particle-particle aggregation, making physical handling of nanoparticles difficult in liquid and dry forms.
4. Small particle size and large surface area readily result in limited drug loading and burst release. These practical problems have to be overcome before nanoparticles can be used clinically or commercially made available.
5.The present work is a step towards development of nanoparticulate drug delivery system, surfacemodification issues, drug loading strategies, release control and potential applications of nanoparticles.
Mechanism of drug delivery via nanoparticle
Nanoparticles exerts its site-specific drug delivery by avoiding the reticuloendothelial system, utilizing enhanced permeability and retention effect and target-specific targeting. Two types of approaches are applied with drug using nanoparticle as carrier15.
a. Surface bound: The drug molecules are adhered to the surface of nanoparticles
b. Core bound: In such methodology the drug particles are concentrated to the matrix of the nanoparticle and carried to the target in the body. Drugs can be loaded onto Nanoparticles by adding them to a solution that contains previously prepared Nanoparticles or by adding them to the reaction mixture during the polymerization process. Nature of interaction of nanoparticle to the drug may be chemical, surface adsorption, and no binding or interaction at all. The amount
of bound drug and the type of interaction of drug and Nanoparticles depend on the chemical structure of the drug and the polymer and the conditions of drug loading15.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Types of Nanoparticles14
The classes of nanoparticles listed below are all very general and multi-functional; however, some of their basic properties and current known uses in nanomedicine are described here.
1) Solid lipid nanoparticles (SLNs)
2) Liposomes
3) Nanostructured lipid carriers (NLC)
4) Fullerenes
5) Nanoshells
6) Quantum dots (QD)
7) Super paramagnetic nanoparticles
Solid lipid nanoparticles (SLNs)
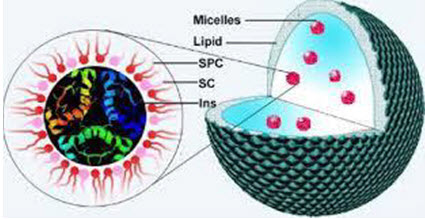
Figure No. 3: Solid Lipid Nanoparticles
SLNs mainly comprise lipids that are in solid phase at the room temperature and surfactants for emulsification, the mean diameters of which range from 50 nm to 1000 nm for colloid drug delivery applications SLNs offer unique properties such as small size, large surface area, high drug loading, the interaction of phases at the interfaces, and are attractive for their potential to improve performance of pharmaceuticals, neutraceuticals and other materials The typical methods of preparing SLNs include spray drying high shear mixing ultra-sonication and high pressure homogenization (HPH) Solid lipids utilized in SLN formulations include fatty acids (e.g. palmitic acid, decanoic acid, and behenic acid), triglycerides (e.g. trilaurin, trimyristin, and tripalmitin), steroids (e.g. cholesterol), partial glycerides (e.g. glyceryl monostearate and gylceryl behenate) and waxes (e.g. cetyl palmitate).
Several types of surfactants are commonly used as emulsifiers to stabilize lipid dispersion, including soybean lecithin,phosphatidylcholine, poloxamer 188, sodium cholate, and sodium glycocholate Advantages of these solid lipid nanoparticles (SLN) are the use of physiological lipids, the avoidance of organic solvents in the preparation process, and a wide potential application spectrum (dermal, oral, intravenous). Additionally, improved bioavailability, protection of sensitive drug molecules from the environment (water, light) and controlled and/or targeted drug release and improved stability of pharmaceuticals, feasibilities of carrying both lipophilic and hydrophilic drugs and most lipids being biodegradable SLNs possess a better stability and ease of upgradability to production scale as compared to liposomes. This property may be very important for many modes of targeting. SLNs form the basis of colloidal drug delivery systems, which are biodegradable and capable of being stored for at least one year.
Liposomes
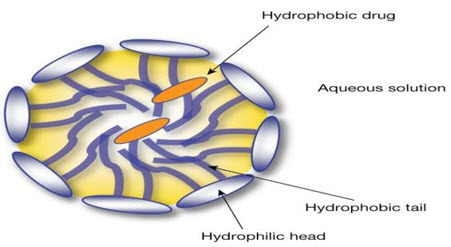
Figure No. 2: Structure of Liposome
Liposomes are vesicular structures with an aqueous core surrounded by a hydrophobic lipid bilayer, created by the extrusion of phospholipids. Phospholipids are GRAS (generally recognized as safe) ingredients, therefore minimizing the potential for adverse effects. Solutes, such as drugs, in the core cannot pass through the hydrophobic bilayer however hydrophobic molecules can be absorbed into the bilayer, enabling the liposome to carry both hydrophilic and hydrophobic molecules. The lipid bilayer of liposomes can fuse with other bilayers such as the cell membrane, which promotes release of its contents, making them useful for drug delivery and cosmetic delivery applications. Liposomes that have vesicles in the range of nanometers are also called nanoliposomes. Liposomes canvary in size, from 15 nm up to several lm and can have either a single layer (unilamellar) or multiple phospholipid bilayer membranes (multilamellar) structure. Unilamellar vesicles (ULVs) can be further classified into small unilamellar vesicles (SUVs) and large unilamellar vesicles (LUVs) depending on their size range. The unique structure of liposomes, a lipid membrane surrounding an aqueous cavity, enables them to carry both hydrophobic and hydrophilic compounds without chemical modification. In addition, the liposome surface can be easily functionalized with ‘stealth’ material to enhance their in vivo stability or targeting ligands to enable preferential delivery of liposomes. These versatile properties of liposomes made them to be used as potent carrier for various drugs like antibacterials, antivirals, insulin, antineoplastics and plasmid DNA.
Nanostructured lipid carriers (NLC)14:
Figure. no.3: Nanostructured lipid carriers
Nanostructured Lipid Carriers are produced from blend of solid and liquid lipids, but particles are in solid state at body temperature. Lipids are versatile molecules that may form differently structured solid matrices, such as the nanostructured lipid carriers (NLC) and the lipid drug conjugate nanoparticles (LDC) that have been created to improve drug loading capacity. The NLC production is based on solidified emulsion (dispersed phase) technologies. NLC can present an insufficient loading capacity due to drug expulsion after polymorphic transition during storage, particularly if the lipid matrix consists of similar molecules. Drug release from lipid particles occurs by diffusion and simultaneously by lipid particle degradation in the body. In some cases it might be desirable to have a controlled fast release going beyond diffusion and degradation. Ideally this release should be triggered by an impulse when the particles are administered. NLCs accommodate the drug because of their highly unordered lipid structures. A desired burst drug release can be initiated by applying the trigger impulse to the matrix to convert in a more ordered structure. NLCs of certain structures can be triggered this way. NLCs can generally be applied where solid nanoparticles possess advantages for the delivery of drugs. Major application areas in pharmaceutics are topical drug delivery, oral and parenteral (subcutaneous or intramuscular and intravenous) route. LDC nanoparticles have proved particularly useful for targeting water-soluble drug administration. They also have applications in cosmetics, food and agricultural products. These have been utilized in the delivery of anti-inflammatory compounds, cosmetic preparation, topical cortico therapy and also increase bioavailability and drug loading capacity.
Fullerene

Figure No. 4: Endohedral Aza[60]fullerenes
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buck balls, and cylindrical ones are called carbon nanotubes or buck tubes. Fullerenes are similar in structure to the graphite, which is composed of stacked grapheme sheets of linked hexagonal rings, additionally they may also contain pentagonal (or sometimes heptagonal) rings to give potentially porous molecules. Buckyballclusters or buck balls composed of less than 300 carbon atoms are commonly known as endohedral fullerenes and include the most common fullerene, buckminsterfullerene, C60.
Mega tubes are larger in diameter than nano tubes and prepared with walls of different thickness which is potentially used for the transport of a variety of molecules of different sizes Nano ‘‘onions’’ are spherical particles based on multiple carbon layers surrounding a buck ball core which are proposed for lubricants. These properties of fullerenes hold great promise in health and personal care application.
Nanoshells

Figure No. 5: Nanoshells
Nanoshells are also notorious as core-shells, nanoshells are spherical cores of a particular compound (concentric particles) surrounded by a shell or outer coating of thin layer of another material, which is a few 1–20 nm nanometers thick Nanoshell particles are highly functional materials show modified and improved properties than their single component counterparts or nanoparticles of the same size. Their properties can be modified by changing either the constituting materials or core-to-shell ratio Nanoshell materials can be synthesized from semiconductors (dielectric materials such as silica and polystyrene), metals and insulators. Usually dielectric materials such as silica and polystyrene are commonly used as core because they are highly stable. Metal nanoshells are a novel type of composite spherical nanoparticles consisting of a dielectric core covered by a thin metallic shell which is typically gold. Nanoshells possess highly favorable optical and chemical properties for biomedical imaging and therapeutic applications. Nanoshells offer other advantages over conventional organic dyes including improved optical properties and reduced susceptibility to chemical/thermal denaturation. Furthermore, the same conjugation protocols used to bind biomolecules to gold colloid are easily modified for nanoshells .When a nanoshell and polymer matrix is illuminated with resonant wavelength, nanoshells absorb heat and transfer to the local environment. This causes collapse of the network and release of the drug. In core shell particles-based drug delivery systems either the drug can be encapsulated or adsorbed onto the shell surface. The shell interacts with the drug via a specific functional group or by electrostatic stabilization method. When it comes in contact with the biological system, it directs the drug. In imaging applications, nanoshells can be tagged with specific antibodies for diseased tissues or tumors.
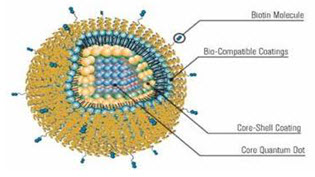
Figure No. 6: Quantum Dots with Coatings
The quantum dots are semiconductor nanocrystals and core shell nanocrystals containing interface between different semiconductor materials. The size of quantum dots can be continuously tuned from 2 to 10 nm, which, after polymer encapsulation, generally increases to 5–20 nm in diameter. Particles smaller than 5 nm are quickly cleared by renal filtration. Semiconductor nanocrystals have unique and fascinating optical properties; become an indispensable tool in biomedical research, especially for multiplexed, quantitative and long-term fluorescence imaging and detection. QD core can serve as the structural scaffold, and the imaging contrast agent and small molecule hydrophobic drugs can be embedded between the inorganic core and the amphiphilic polymer coating layer. Hydrophilic therapeutic agents including small interfering RNA (siRNA) and antisense oligodeoxynucleotide (ODN)) and targeting biomolecules such as antibodies, peptides and aptamers can be immobilized onto the hydrophilic side of the amphiphilic polymer via either covalent or non-covalent bonds. This fully integrated nanostructure may behave like magic bullets that will not only identify, but bind to diseased cells and treat it. It will also emit detectable signals for real-time monitoring of its trajectory.
Superparamagnetic nanoparticles14:
Super paramagnetic molecules are those that are attracted to a magnetic field but do not retain residual magnetism after the field is removed. Nanoparticles of iron oxide with diameters in the 5–100 nm range have been used for selective magnetic bio separations. Typical techniques involve coating the particles with antibodies to cell-specific antigens, for separation from the surrounding matrix. The main advantages of superparamagnetic nanoparticles are that they can be visualized in magnetic resonance imaging (MRI) due to their paramagnetic properties; they can be guided to a location by the use of magnetic field and heated by magnetic field to trigger the drug release. Super paramagnetic nanoparticles belong to the class of inorganic based particles having an iron oxide core coated by either inorganic materials (silica, gold) and organic (phospholipids, fatty acids, polysaccharides, peptides or other surfactants and polymers). In contrast to other nanoparticles, superparamagnetic nanoparticles based on their inducible magnetization, their magnetic properties allow them to be directed to a defined location or heated in the presence of an externally applied AC magnetic field. These characteristics make them attractive for many applications, ranging from various separation techniques and contrast enhancing agents for MRI to drug delivery systems, magnetic hyperthermia (local heat source in the case of tumor therapy), and magnetically assisted transfection of cells. Already marketable products, so-called beads, are micron sized polymer particles loaded with SPIONs. Such beads can be functionalized with molecules that allow a specific adsorption of proteins or other biomolecules and subsequent separation in a magnetic field gradient for diagnostic purposes.
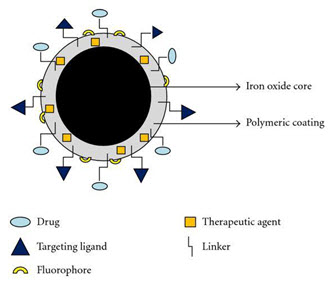
Figure No. 4: Super Paramagnetic Nanoparticle
More interesting applications, like imaging of single cells or tumors, delivery of drugs or genes, local heating and separation of peptides, signalling molecules or organelles from a single living cell or from a living (human) body are still subjects of intensive research. The transdisciplinarity of basic and translational research carried out in superparamagnetic nanoparticles during the last decades lead to a broad field of novel applications for superparamagnetic nanoparticles.
Preparation of Nanoparticles16
Nanoparticles can be prepared from a variety of materials such as proteins, polysaccharides and synthetic polymers. The selection of matrix materials is dependent on many factors including17:
(a) size of nanoparticles required.
(b)inherent properties of the drug, e.g., aqueous solubility and stability.
(c) surface characteristics such as charge and permeability.
(d) degree of biodegradability, biocompatibility and toxicity.
(e) Drug release profile desired.
(f) Antigenicity of the final product.
Nanoparticles have been prepared most frequency by three methods:
(1) dispersion of preformed polymers
(2) polymerization of monomers; and
(3)ionic gelation or coacervation of hydrophilic polymers.
However, other methods such as supercritical fluid technology18and particle replication in non-wetting templates(PRINT)19have also been described in the literature for production of nanoparticles. The latter was claimed to have absolute control of particle size, shape and composition, which could set an example for the future mass production of nanoparticles in industry
Dispersion of preformed polymers:
preformed polymers is a common technique used to prepare biodegradable nanoparticles from poly (lactic acid) (PLA); poly (D,L-glycolide),PLG; poly (D, L-lactide-co-glycolide) (PLGA) and poly (cyanoacrylate) (PCA),20-22. This technique can be used in various ways as described below.
Solvent evaporation method:
In this method, the polymer is dissolved in an organic solvent such as dichloromethane, chloroform or ethyl acetate which is also used as the solvent for dissolving the hydrophobic drug. The mixture of polymer and drug solution is then emulsified in an aqueous solution containing a surfactant or emulsifying agent to form oil in water (o/w) emulsion. After the formation of stable emulsion, the organic solvent is evaporated either by reducing the pressure or by continuous stirring. Particle size was found to be influenced by the type and concentrations of stabilizer, homogenizer speed and polymer concentration23. In order to produce small particle size, often ahigh-speed homogenization or ultrasonicationmay be employed24.
Spontaneous emulsification or solvent diffusion method:
This is a modified version of solventevaporation method25. In this method, the watermiscible solvent along with a small amount of the water immiscible organic solvent is used as an oil phase. Due to the spontaneous diffusion of solvents an interfacial turbulence is createdbetween the two phases leading to the formation of small particles. As the concentration of water miscible solvent increases, a decrease in the size of particle can be achieved. Both solvent evaporation and solvent diffusion methods can be used for hydrophobic or hydrophilic drugs. In the case of hydrophilic drug, a multiple w/o/w emulsion needs to be formed with the drug dissolved in the internal aqueous phase between the two phases leading to the formation of small particles. As the concentration of water miscible solvent increases, a decrease in the size of particle can be achieved. Both solvent evaporation and solvent diffusion methods can be used for hydrophobic or hydrophilic drugs. In the case of hydrophilic drug, a multiple w/o/w emulsion needs to be formed with the drug dissolved in the internal aqueous phase.
Polymerization method
In this method, monomers are polymerized to form nanoparticles in an aqueous solution. Drug is incorporated either by being dissolved in the polymerization medium or by adsorption onto the
Nanoparticles after polymerization completed. The nanoparticle suspension is then purified to remove various stabilizers and surfactants employed for polymerization by ultracentrifugation and re-suspending the particles in an isotonic surfactant-free medium.
This technique has been reported for making polybutylcyanoacrylate or poly (alkylcyanoacrylate) nanoparticles26-27.
Nanocapsule formation and their particle size depends on the concentration of the surfactants and stabilizers used 28.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Coacervation or ionic gelation method
Much research has been focused on the preparation of nanoparticles using biodegradable hydrophilic polymers such as chitosan, gelatin and sodium alginate. Calvo and co-workers developed a method for preparing hydrophilic chitosan nanoparticles by ionic gelation 29-30. The method involves a mixture of two aqueous phases, of which one is the polymer chitosan, a di-block co-polymer ethylene oxide or propylene oxide (PEO-PPO) and the other is a polyanion sodium tripolyphosphate. In this method, positively charged amino group of chitosan interacts with negative charged tripolyphosphate to form coacervates with a size in the range of nanometer. Coacervates are formed as a result of electrostatic interaction between two aqueous phases, whereas, ionic gelation involves the due to ionic interaction conditions at room temperature.
Production of nanoparticles using
supercritical fluid technology16
Conventional methods such as solvent extraction-evaporation, solvent diffusion and organic phase separation methods require the use of organic solvents which are hazardous tothe environment as well as to physiological systems. Therefore, the supercritical fluid technology has been investigated as an alternative to prepare biodegradable micro- and nanoparticles because supercritical fluids are environmentally safe 16. A supercritical fluid can be generally defined as a solvent at a temperature above its critical temperature, at which the fluid remains a single phase regardless of pressure 16. Supercritical CO2 (SC CO2) is the most widely used supercritical fluid because of its mild critical conditions (Tc = 31.1 °C, Pc = 73.8 bars), nontoxicity, non-flammability, and low price. The most common processing techniques involving supercritical fluids are supercritical anti-solvent (SAS) and rapid expansion of critical solution (RESS). The process of SAS employs a liquid solvent, eg methanol, which is completely miscible with the supercritical fluid (SC CO2), to dissolve the solute to be micronized; at the process conditions, because the solute is insoluble in the supercritical fluid, the extract of the liquid solvent by supercritical fluid leads to the instantaneous precipitation of the solute, resulting the formation of nanoparticles18. Thote and Gupta (2005) reported the use of a modified SAS method for formation of hydrophilic drug dexamethasone phosphate drug nanoparticles for microencapsulation purpose 16. RESS differs from the SAS process in that its solute is dissolved in a supercritical fluid (such as supercritical methanol) and then the solution is rapidly expanded through a small nozzle into a region lower pressure 16 , Thus the solvent power of supercritical fluids dramatically decreases and the solute eventually precipitates. This technique is clean because the precipitate is basically solvent free. RESS and its modified process have been used for the product of polymeric nanoparticles16. Supercritical fluid technology technique, although environmentally friendly and suitable for mass production, requires specially designed equipment and is more expensive.
Evaluation of Nanoparticles32
The nanoparticles are generally evaluated for the following:
1) Size and morphology
2) Specific surface
3) Surface charge and electrophoretic mobility
4) Density of nanoparticles
5) Molecular weight
6) Nanoparticle recovery and drug incorporation efficiency
7) In vitro release
1)Size and morphology:
Size of nanoparticle is determined by
a) Photon correlation spectroscopy(PCM)
b) Electron microscopy(EM): This include
1)Scanningelectron microscopy(SEM)
2)Transmission electron microscopy(TEM)
3)Freez fraction electron microscopy(FFEM)
c) Atomic force microscopy Electron microcopy is less time consuming, FFEM give the internal morphology of particles.TEM and FFEM provide differentiation among nanoparticle and nanocapsules and emulsion droplets. All the particles are coated with gold as they are non-conductive (thickness of gold coast is 30-50nm). Thus determined size should be denoted as gold-coated particle size. Atomic force microscopy(AFM) is an advanced nanoscopic technique and used for characterization of PLA nanosphere.Mercury porositometery is used to measure size of nanoparticles.
2)Specific surface:
Specific surface of freez dried nanoparticles is determined with sorptometer and it is calculated by using the formula:
A= 6/ D.d
Where
A= Specific surface
D= Density
d= Diameter of the particle
3)Surface charge and electrophoretic mobility:
Surface charge of nanoparticles can be determined by measuring the velocity of particle in an electronic field. It can also be measured as electrophoretic mobility. The electrophoretic mobility is determined in phosphate saline buffer & human serum. Laser Doppler anemometry or velocimetry is widely used techniques for determination of velocities. Phosphate saline buffer (PH-7.4) reduces the charge value of nanoparticles, zeta potential can be obtained by measuring the electrophoretic mobility applying Helmholtz-Smoluchowski equation.
4)Density of nanoparticles:
Density of nanoparticles is determined with helum or air using a gas pychnometer
5) Molecular weight:
Molecular weight of polymer and its distribution in the matrix can be evaluated by Gel permeation chromatography.
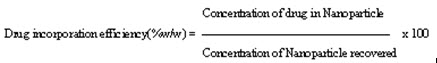
6) Nanoparticle recovery and drug incorporation efficiency:
Nanoparticle recovery or Nanoparticle yield can be calculated using equation:

Drug incorporation efficiency or drug content can be calculated using equation:
7) In vitro release:
In vitro release is determined by using dialysis diffusion cell or nodified ultrafiltration technique and phosphate buffer is used for it.Donar and receptor chambers are separated by milipore membrane. Donar compartment have nanoparticles along with phosphate buffer, while the receptor compartment contain only buffer solution.The receptor compartment is assayed for drug release at various time interval.
Another types of methods for evaluation
Drug Entrapmaent Efficiency31
The nanoparticles were separated from the aqueous medium by ultracentrifugation at 10,000 rpm for 30 min at 5c. Then the resulting supernatant solution was decanted and dispersed intophosphate buffer saline pH 7.4. Thus the procedure was repeated twice to remove the unentrapped drug molecules completely. The amount of drug entrapped in the nanoparticles was determined as the difference between the total amount of drug used to prepare the nanoparticles and the amount of drug present in the aqueous medium. Drug Entrapment efficiency (%) = Amount of released from the lysed nanoparticleX 100 Amount of drug Initially taken to prepare the nanoparticles
Particle Shape31 The nanoparticles were subjected to microscopic examination (SEM) for characterization size. The nanosuspension was characterized by SEM before going for evaluation; the nanosuspension was lyophilized to form solid particles. The solid particles were coated with platinum alloy using a sputter coater.
Particle size31
Particle size and size distribution are the most important characteristics of nanoparticle systems. They determine the in vivo distribution, biological fate, toxicity and targeting ability of nanoparticle system. In addition, they can also influence the drug loading, drug release and stability of nanoparticles. Currently, the faster and most routine method of determining particle size is by photon-correlation spectroscopy or dynamic light scattering. The results obtained by photon-correlation spectroscopy are usually verified by scanning or transmission electron microscopy (SEM or TEM).
Zeta potential31
The Zeta potential of a nanoparticle is commonly used to characterized the surface charge property of nanoparticles. It reflects the electrical potential of particles and is influenced by the composition of the particle and the medium in which it is dispersed. Nanoparticles with a zeta potential above (±) 30 mV have been shown to be stable in suspension, as the surface charge prevents aggregation of the particles.
Nanoparticle Properties and Safety31
The nanoparticles are likely to be unsafe for the biological system. The research on toxicity of nanoparticles indicates that some of these products may enter the human body and become toxic at the cellular level in the tissues and organs. The impact of nanoparticles interactions with the body is dependent on their size, chemical composition, surface structure, solubility, shape and how the individual nanoparticles accumulate together. Due to small size and hence higher specific surface area of the nanoparticles, these can easily bind with the transport toxic pollutants, which when inhaled can cause a number of pulmonary disease in mammals. Inhaled nanoparticles have the ability to translate in the body as much the nanoparticles enter the body these can travel freely in the blood throughout the body and reach the organ like liver or brain. It can get deeper into the lungs and bloodstream may cross the blood brain barrier. Skin contact could easily occur during handling of the nanoparticles. Fullerencs and bucky balls, which are known to attract electrons, cause generation of damaging free radicals. Nan toxicity studies of carbon-based materials as well as quantum dots have been conducted. Literature shows that lowsolubility ultrafine particles are more toxic than larger particles on a mass for mass basis31.
STABILITY UPON STORAGE OF THE NANOPARTICLES33
The necessity for and degree of purification are dependent on the final purpose of the formulation developed. The most commonly reported procedures are gel filtration, ultracentrifugation, dialysis, and, recently, cross-flow filtration. Another requirement relates to the sterilization of the formulation To prevent a complete precipitation, it is necessary to incorporate some additives Lyophilization (freeze-drying) seems to be a highly stabilizing process This technique involves the freezing of the suspension and subsequent elimination of its water content by sublimation under reduced pressure Freezing is the most aggressive step of the freeze-drying operation for colloidal operations. It is thus important to improve thenanoparticle resistance by addition of a cryoprotectant to avoid alteration of the suspension[54]. Sometimes cryoprotectants like glucose, trealose, mannitol, and sorbitol were added to ensure redispersibilty or to allow vitrification of the suspension during the cooling and to avoid crystallization of the liquid suspension33.
NANOPARTICLES AND THEIR HEALTH EFFECTS32
Nanoparticles have exceptional physical, chemical and electrical properties. What about their biological properties and interactions with the human body? Do they present a health risk for the workers who produce, handle, transform or use them? There are two major reasons for modifying nanoparticle surfaces. First, a surface coating is frequently used to prevent aggregation of particles, but little data is available on the toxicity of these coated nanoparticles. Second, many modifications have been made to nanoparticle surfaces to modify their behavior in the human body and develop new medications several studies on the toxicity of specific nanoparticles (fullerenes, carbon nanotubes, quantum dots) are available.
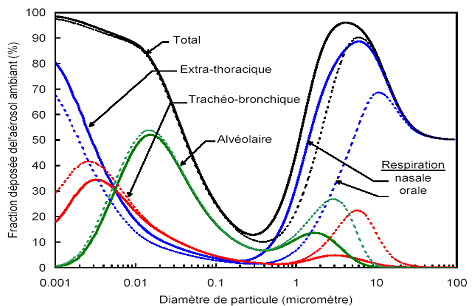
Figure: 20 Prediction of total and regional deposits of particles in the airway according to particle size (41). Reproduced with the authorization of INRS-France
Quantitative assessment of the risk of exposure to nanoparticles
The international experts assembled by the European Commission are unanimous that the potential deleterious effect of nanoparticles cannot be predicted from the toxicity of bulk materials of the same chemical composition but of greater size.
The risk assessment cannot be established precisely, since the dose-response relationships are insufficiently known. For most of the particles that can become airborne and breathed in, the primary concern is the potential damage to the respiratory system, which represents the most likely absorption route in the work environment.
The solubility of the particles directly affects their toxicity and the way they must be assessed or analyzed. In the presence of soluble particles, the entire mass deposited in the pulmonary air passages will quickly become available by dissolution in the biological fluids. This mechanism is well known for particles of larger dimensions. It is the same for nanoparticles. For soluble particles, the assessment of the total mass concentration of these particles thus will become a good indicator of their toxicity. However, insoluble or low solubility particles will retain their form and expose their surface to the host organism. It then becomes important to document the specific toxicity of these insoluble or low solubility nanoparticles. In the absence of adequate knowledge, attention should also be paid to the cutaneous system and the possibility of ingestion of nanoparticles, particularly by adopting strictly hygienic safety measures. To assess the potential human health effects of nanoparticles, it is important to develop knowledge that can provide answers to several questions. Specifically, it is essential to document how nanoparticles are absorbed into the human body by several routes (pulmonary, cutaneous and gastrointestinal), how nanoparticles are distributed in the body (blood, lymphatic system), the organs in which nanoparticles tend to accumulate significantly (lungs, brain, kidneys, liver…), and how nanoparticles are metabolized and then eliminated32.
APPLICATIONS OF NANOPARTICALS
Tumor targeting using Nano particulate delivery system31
The rational of using nanoparticles for tumor targeting is based on
(1) nanoparticles will be able to deliver a concentrate dose of drug in the vicinity of the tumor targets via the enhanced permeability and retention effect or active nanoparticles. (2) Nanoparticles will reduce the drug exposure of health tissues by limiting drug distribution to target organ. Verdun et al demonstrated in mice treated with doxorubicin incorporated into poly (isohexylcynoacrylate) nanospheres that higher concentration of doxorubicin manifested in the liver, spleen and lungs than in mice treated with free doxorubicin.
Nanoparticles for Gene delivery31
Polynucleotide vaccines work by delivering genes encoding relevant antigens to host cells where they are expressed, producing the antigenic protein within the vicinity of professional antigen presenting cells to initiate immune response. Such vaccines produce both humoral and cell-mediated immunity because intracellular production of protein, as opposed to extracellular deposition, stimulates both arms of the immune system.
Nanotechnology in Medicine Application:
Anti-Microbial Techniques31
One of the earliest nanomedicine applications was the use of nanocrystalline silver which is as an antimicrobial agent for the treatment of wounds, A nanoparticle cream has been shown to fight staph infections. The nanoparticles contain nitric oxide gas, which is known to kill bacteria. Studies on mice have shown that using the nanoparticle cream to release nitric oxide gas at the site of staph abscesses significantly reduced the infection. Burn dressing that is coated with nanocapsules containing antibotics. If an infection starts the harmful bacteria in the wound causes the nanocapsules to break open, releasing the antibotics. This allows much quicker treatment of an infection and reduces the number of times a dressing has to be changed. A welcome idea in the early study stages is the elimination of bacterial infections in a patient within minutes, instead of delivering treatment with antibiotics over a period of weeks.
Nanotechnology in Medicine Application31:
Cell Repair Nanorobots could actually be programmed to repair specific diseased cells, functioning in a similar way to antibodies in our natural healing processes. Read about design analysis for one such cell repair nanorobot in this article: The Ideal Gene Delivery Vector: Chromallocytes, Cell Repair Nanorobots for Chromosome Repair Therapy.
|
Company |
Product |
|---|---|
|
BioDelivery Sciences |
Oral drug delivery of drugs encapuslated in a nanocrystalline structure called a cochleate |
|
CytImmune |
Gold nanoparticles for targeted delivery of drugs to tumors |
|
Invitrogen |
Qdots for medical imaging |
|
Smith and Nephew |
Antimicrobial wound dressings using silver nanocrystals |
|
Luna Inovations |
Bucky balls to block inflammation by trapping free radicals |
|
NanoBio |
Nanoemulsions for nasal delivery to fight viruses (such as the flu and colds) or through the skin to fight bacteria |
|
NanoBioMagnetics |
Magnetically responsive nanoparticles for targeted drug delivery and other applications |
TABLE:Nanotechnology in Medicine: Company Directory
Future Opportunities and Challenges
Nanoparticles and nanoformulations have already been applied as drug delivery systems with great success; and nanoparticulate drug delivery systems have still greater potential for many applications, including anti-tumor therapy, gene therapy, and AIDS therapy, radiotherapy, in the delivery of proteins, antibiotics, virostatics, vaccines and as vesicles to pass the blood - brain barrier34. Nanoparticles and nanoformulations have already been applied as drug delivery systems with great success; and nanoparticulate drug delivery systems have still greater potential for many applications, including anti tumour therapy, gene therapy, AIDS therapy, radiotherapy, in the delivery of proteins, antibiotics, virostatics, vaccines and as vesicles to pass the blood-brain barrier. Nanoparticles provide massive advantages regarding drug targeting, delivery and release and, with their additional potential to combine diagnosis and therapy, emerge as one of the major tools in nanomedicine. The main goals are to improve their stability in the biological environment, to mediate the bio-distribution of active compounds, improve drug loading, targeting, transport, release, and interaction with biological barriers. The cytotoxicity of nanoparticles or their degradation products remains a major problem, and improvements in biocompatibility obviously are a main concern of future research32. There are many technological challenges to be met, in developing the following techniques32:
a)-Nano-drug delivery systems that deliver large but highly localized quantities of drugs to specific areas to be released in controlled ways; b) -Controllable release profiles, especially for sensitive drugs;
c) -Materials for nanoparticles that are biocompatible and biodegradable;
d) -Architectures / structures, such as biomimetic polymers, nanotubes;
e) -Technologies for self-assembly;
f)- Functions (active drug targeting, on-command delivery, intelligent drug release devices/ bioresponsive triggered systems, self-regulated delivery systems, systems interacting with the body, smart delivery);
g) -Virus-like systems for intracellular delivery;
h) -Nanoparticles to improve devices such as implantable devices/nanochips for nanoparticle release, or multi reservoir drug delivery-chips; i) -Nanoparticles for tissue engineering; e.g. for the delivery of cytokines to control cellular growth and differentiation, and stimulate regeneration; or for coating implants with --nanoparticles in biodegradable polymer layers for sustained release;
j) -Advanced polymeric carriers for the delivery of therapeutic peptide/proteins (biopharmaceutics), And also in the development of: k) -Combined therapy and medical imaging, for example, nanoparticles for diagnosis and manipulation during surgery (e.g. thermotherapy with magnetic particles);
l) -Universal formulation schemes that can be used as intravenous, intramuscular or peroral drugs m) -Cell and gene targeting systems. n) -User-friendly lab-on-a-chip devices for point-of-care and disease prevention and control at home.
o)-Devices for detecting changes in magnetic or physical properties after specific binding of ligands on paramagnetic nanoparticles that can correlate with the amount of ligand. -Better disease markers in terms of sensitivity and specificity.
Marketed Products of Nanomedicine12
1. Nanoparticle
2. Nanocrystal
3. Nanotube
4. Superparamagnetic iron oxide
5. Liposomes
6. Micelle
Some Indian Technologies
1. First produced smart hydrogel nanoparticles for drug delivery applications (US Patent 5847111)
2. Tumor Targeted Delivery of Taxol using nanoparticles (US Patent 6,322,817 )
3. Inorganic Nanoparticles as non-viral vectors for targeted delivery of genes (US Patent 6555376 ); Technology transferred to a California based Pharm Com
4. Once in 48 hours dose Ophthalmic delivery (US Patent 6579519) (Another improved formulation patent on ophthalmic gels is being submitted in India)
5. Oral Insulin Delivery (Patent Pending)
CONCLUSION
Nanoparticles represent a promising drug delivery system of controlled and targeted release. The emergence of nanotechnology is likely to have a significant impact on drug delivery sector, affecting just about every route of administration from oral to injectable. And the payoff for doctors and patients should be lower drug toxicity, reduced cost of treatments, improved bioavailability, and an extension of the economic life of proprietary drugs. This would allow earlier and more personalized diagnosis and therapy, improving the effectiveness of drug treatments and reducing side effects. In addition, nanoparticles are a promising platform technology for the synthesis of molecular-specific contrast agents12. Nanoparticulate systems have great potentials, being able to convert poorly soluble, poorly absorbed and labile biological active substance into promising deliverable drugs. Generally nanoparticle have relatively higher intracellular uptake compared to microparticles and available to a wide range of biological targets due to their small size and relative mobility31.
REFERENCE:
1.N. C Shinde; N.J Keskar; P. D Argade.RJPBCS., 2012, 3, 922.
2.Buzea C, Pacheco II, Robbie K. (2007). Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2 (4): MR17-MR71.
3.Roco MC, Bainbridge WS. (2005). Societal implications of nanoscience and nanotechnology: maximizing human benefit. Journal of Nanoparticle Research. 7 (1): 1-13.
4.Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory's experience. Acc Chem Res 2000; 33: 94-101.
5.Bhadra D, Bhadra S, Jain P, Jain NK. Pegnology: a review of PEG-ylated systems. Pharmazie 2002; 57: 5-29.
6.Kommareddy S, Tiwari SB, Amiji MM. Long-circulating polymeric nanovectors for tumor-selective gene delivery. Technol Cancer Res Treat 2005; 4: 615-25.
7.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res 2005; 22: 1-10.
8.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. (2001). Biodegradable polymeric nanoparticles as drug delivery devices. Journal of controlled release. 70 (1): 1-20.
9.Moghimi SM, Hunter AC, Murray JC. (2001). Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews. 53 (2): 283-318.
10.Lamprecht A, Ubrich N, Yamamoto H, Schäfer U, Takeuchi H, Maincent P, Kawashima Y, Lehr C-M. (2001). Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. Journal of Pharmacology and Experimental Therapeutics. 299 (2): 775-781.
11.Shivakumar H, Gowda D, Krishna R, Das D. (2005). Nanoparticles-Targeting neurotherapeutic agents through the blood brain barrier. INDIAN DRUGS-BOMBAY-. 42 (11): 709.
12.V.B. Kadam*, K.B. Dhanawade, V.A. Salunkhe, A.T. Ubale A. T. Journal of Current Pharma Research 4 (4), 2014, 1318-1335.
13.R.S.M. Krishna. Ind. J. Pharm. Educ. Res., 40, 1 (200) 615-9.
14.R. Sagar, A. Mudshinge. Saudi Pharmaceutical Journal, 19 (2011) 129–141.
15.Mohd Athar1*, Amar Jyoti Das2 Review ArticleAdv. Mater. Rev. 2014, 1(1), 25-37
16.VJ Mohanraj1* and Y Chen2 Research ArticleTropical Journal of Pharmaceutical Research, June 2006; 5 (1): 561-573
17.Kreuter J. Nanoparticles. In Colloidal drug delivery systems, J, K., Ed. Marcel Dekker: New York,1994; pp 219-342.
18.Reverchon E, Adami R. Nanomaterials and supercritical fluids. The Journal of Supercritical Fluids 2006; 37:1-22.
19.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005; 127:10096-10100
20.Kompella UB, Bandi N, Ayalasomayajula SP. Poly (lactic acid) nanoparticles for sustained release of
budesonide. Drug Deliv.Technol. 2001; 1: 1-7.
21.Ravi MN, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials 2004; 25: 1771-1777.
22.Li YP, Pei YY, Zhou ZH, Zhang XY, Gu ZH, Ding J, Zhou JJ, Gao, XJ, PEGylated polycyanoacrylate nanoparticles as tumor necrosis factor-[alpha] carriers. J Control Release 2001; 71: 287-296.
23.Kwon, HY, Lee JY, Choi SW, Jang Y, Kim JH. Preparation of PLGA nanoparticles containing estrogen by emulsification-diffusion method. Colloids Surf. A: Physicochem. Eng. Aspects 2001;182: 123-130.
24.Zambaux M, Bonneaux F, Gref R, Maincent P, Dellacherie E, Alonso M, Labrude P, Vigneron C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by double emulsion.
25.Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y. Preparation of biodegradable nanoparticles of water-soluble and insoluble drugs with D,Llactide/ glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J. Control. Release 1993; 25: 89-98.
26.Zhang Q, Shen Z, Nagai T. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int. J. Pharm. 2001; 218: 75-80.
27.Boudad H, Legrand P, Lebas G, Cheron M, Duchene D, Ponchel G. Combined hydroxypropyl-[beta]-
cyclodextrin and poly(alkylcyanoacrylate) nanoparticles intended for oral administration of saquinavir. Int J. Pharm. 2001; 218: 113-124.
28.Puglisi G, Fresta M, Giammona G, Ventura CA. Influence of the preparation conditions on poly(ethylcyanoacrylate) nanocapsule formation.Int. J. Pharm. 1995; 125: 283-287.
29.Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoprticles as protein carriers. J. Appl. Polymer Sci. 1997; 63: 125-132.
30.Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997; 14: 1431-1436.
31.Prabhjot Kaur et al reaserch artical IJRPC 2012, 2(3)
32.https://www.pharmatutor.org/articles/novel-drug-delivery-system?page=4
33.Sarika Anand Jadhav*, Prof. Prashant Patil and Dr. Ramesh Kalkotwar Vol 5, Issue 10, 2016 wjpps. research article of NANOPARTICLES AS – PARTICULATE DRUG DELIVERY SYSTEM wjpps_.
34.Kedar Prasad Meena*, J.S. Dangi, P K Samal and Manoj Kumars review on
Nanoparticles Technology and Recent Advances in Novel Drug Delivery systems 2011; 1 (1): 1-5.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









