ABOUT AUTHORS:
*Banoj kumar Mahanta, Pankaj Mohanta
Kanak manjari institute of pharmaceutical sciences,
rourkela, Odisha, india
*banoj12@gmail.com
ABSTRACT
The purpose of this study was to develop formulations of mucoadhesive patches of Acetaminophen. The patches were prepared by the solvent casting method using Eudragit RL 100, Polyvinyl alcohol and Carbopol. 6 different formulations were made. The patches were found to be smooth in appearance, uniform in thickness, weight uniformity, drug content, swelling behaviour, and surface pH.
REFERENCE ID: PHARMATUTOR-ART-1865
INTRODUCTION
Amongst the various routes of drug delivery, oral route is perhaps the most preferred to the patient and the clinician alike. However, peroral administration of drugs has disadvantages such as hepatic first pass metabolism and enzymatic degradation within the GI tract, that prohibit oral administration of certain classes of drugs such as peptides and proteins. Consequently, other absorptive mucosa are considered as potential sites for drug administration. Transmucosal routes of drug delivery (i.e., the mucosal linings of the nasal, rectal, vaginal, ocular, and oral cavity) offer distinct advantages over peroral administration for systemic drug delivery. These advantages include possible bypass of first pass effect, avoidance of presystemic elimination within the GI tract, and depending on the particular drug, a better enzymatic flora for drug absorption. Amongst the various routes of administration tried so far in the novel drug delivery systems, localized drug delivery to tissues of the oral cavity has been investigated for the treatment of periodontal disease, bacterial and fungal infection. Over the decades mucoadhesion has become popular for its potential to optimize localized drug delivery, by retaining a dosage form at the site of action (e.g. within the gastrointestinal tract) or systemic delivery by retaining the formulation in intimate contact with the absorption site (e.g. buccal cavity). Well defined bioadhesion is the ability of a material (synthetic or biological) to adhere to a biological tissue for an extended period of time. The biological surface can be epithelial tissue or it can be the mucus coat on the surface of a tissue. If adhesion is to a mucous coat, the phenomenon is referred to as mucoadhesion.
The use of mucoadhesive polymers in buccal drug delivery has a great applications. Various mucoadhesive devices, including tablets, films, patches, disks, strips, ointments and gels, have recently been developed.
However, buccal patch offer greater flexibility and comfort than the other devices. In addition, a patch can circumvent the problem of the relatively short residence time of oral gels on mucosa, since the gels are easily washed away by saliva. Buccal route of drug delivery provides the direct access to the systemic circulation through the jugular vein by passing the first pass hepatic metabolism leading to high bioavailability. Other advantages such as excellent accessibility, low enzymatic activity, suitability for drugs or excipients that mildly and reversibly damage or irritate the mucosa, painless administration, easy withdrawal, facility to include permeation enhancer/ enzyme inhibitor or pH modifier in the formulation, versatility in designing as multidirectional or unidirectional release system for local or systemic action.
BUCCAL DRUG DELIVERY SYSTEM
A delivery system designed to deliver drugs systemically or locally via buccal mucosa. Buccal delivery refers to the drug release which can occur when a dosage form is placed in the outer vestibule between the buccal mucosa and gingival.
MECHANISM OF BUCCAL ABSORPTION
Buccal drug absorption occurs by passive diffusion of the nonionized species, A process governed primarily by a concentration gradient, through the intercellular spaces of the epithelium. The passive transport of non-ionic species across the lipid membrane of the buccal cavity is the primary transport mechanism. The buccal mucosa has been said to be a lipoidal barrier to the passage of drugs, as is the case with many other mucosal membrane and the more lipophilic the drug molecule, the more readily it is absorbed. The dynamics of buccal absorption of drugs could be adequately described by first order rate process. Several potential barriers to buccal drug absorption have been identified. Dearden and Tomlison (1971) pointed out that salivary secretion alters the buccal absorption kinetics from drug solution by changing the concentration of drug in the mouth. The linear relationship between salivary secretion and time is given as follows:
Where,
-dm/dt = KC/ViVt
M - Mass of drug in mouth at time t
K - Proportionality constant
C - Concentration of drug in mouth at time
Vi - The volume of solution put into mouth cavity and Vt - Salivary secretion rate
ADVANTAGES OF BUCCAL PATCHES
1. The oral mucosa has a rich blood supply. Drugs are absorbed from the oral cavity through the oral mucosa, and transported through the deep lingual or facial vein, internal jugular vein and braciocephalic vein into the systemic circulation.
2. Buccal administration, the drug gains direct entry into the systemic circulation thereby bypassing the first pass effect. Contact with digestive fluids of gastrointestinal tract is avoided which might be unsuitable for stability of many drugs like insulin or other proteins, peptides and steroids. In addition, the rate of drug absorption is not influenced by food or gastric emptying rate.
3. The area of buccal membrane is sufficiently large to allow a delivery system to be placed at different occasions, additionally; there are two areas of buccal membranes per mouth, which would allow buccal drug delivery systems to be placed, alternatively on the left and right buccal membranes.
4. Buccal patch has been well known for its good accessibility to the membranes that line the oral cavity, which makes application painless and with comfort.
5. Patients can control the period of administration or terminate delivery in case of emergencies. The buccaldrug delivery systems easily administered in the buccal cavity. The novel buccal dosage forms exhibits better patient compliance.
MATERIALS USED
A. Active ingredient – Acetaminophen.
B. Polymers : Polyvinyl alcohol, Carbopol and Eudragit RL-100.
C. Solvent: Ethyl Alcohol.
D. Plasticizer: Propylene glycol.
FORMULATION
The buccal mucoadhesive patches of Acetaminophen BPC1 was prepared by solvent casting method using film forming polymers for the patches mentioned in table 1.Eudragit RL 100 polymer was weighed accurately and dissolved in ethanol to prepare 10%w/v. Polyvinyl Alcohol was weighed accurately and dissolved in water to prepare 2%w/v. Carbopol was weighed accurately and dissolved in ethanol to prepare 1%w/v.The drug solution is prepared using acetaminophen in ethanol. To prepare BPC1 10ml of eudragit solution was taken into the beaker and 0.05% of tween 80was added to it ,mixed well on a magnetic stirrer. To the above solution 10 ml of Polyvinyl Alcohol solution and 10 ml of Carbopol solutionwere added and mixed well thoroughly. To this mixture 2 ml of Propylene Glycol was added and mixed well on magnetic stirrer at low rpm for nearly 1 hour to get clear homogenous mixture, bubble free solution. To this mixture, a drug solution corresponding to 50 mg was added and mixed thoroughly to obtain uniform distribution of the drug . This solution was then poured into specially fabricated Teflon coated circular disc. The patch was then dried at room temperature for nearly 2 hour and were further dried for 18 hour at 40°C in hot air oven. Finally patches was vaccum dried for 4 hour at room temperature in a vaccum dessicator.
After careful examination, The dried patch was removed checked for any imperfection or any air bubbles and cut in 2 cm diameter patch using a specially fabricated circular stainless steel cutter. The patch was laminated on one side with water impermeable backing layer. The sample was packed in aluminium foil and stored in glass container. Similarly BPC2 ,BPC3 , BPC4, BPC5 were prepared.
TABLE NO-1
|
INGREDIENTS |
BUCCAL PATCH CODE |
||||
|
BPC1 |
BPC2 |
BPC3 |
BPC4 |
BPC5 |
|
|
EUDRAGIT RL 100 (10%) w/v |
10 ml |
8.5 ml |
7.5 ml |
6.6 ml |
6 ml |
|
POLYVINYL ALCOHOL (2%)w/v |
10 ml |
8.5 ml |
7.5 ml |
9.9 ml |
12 ml |
|
CARBOPOL(1%)w/v |
10 ml |
13 ml |
15 ml |
13.3 ml |
12 ml |
|
PROPYLENE GLYCOL |
2 ml |
2 ml |
3.5 ml |
3 ml |
4 ml |
|
ACETAMINOPHEN |
50 mg |
50 mg |
50 mg |
50 mg |
50 mg |

FIG 1- Homogenous mixture formation formation by Magnetic stirrer
Evaluation of Buccal patch
1. Surface pH
Buccal patches are left to swell for 2 hr on the surface of an agar plate. The surface pH is measured by means of a pH paper placed on the surface of the swollen patch
2. Thickness measurements
The thickness of each film is measured at five different locations (centre and four corners) using an electronic digital micrometer
3. Swelling study
Buccal patches are weighed individually (designated as W1), and placed separately in 2% agar gel plates, incubated at 37°C ± 1°C, and examined for any physical changes. At regular 1-hour time intervals until 3 hours, patches are removed from the gel plates and excess surface water is removed carefully using the filter paper [11]. The swollen patches are then reweighed (W2) and the swelling index (SI) is calculated using the following formula.
SI = (W2-W1) x100 / W1
4.Thermal analysis study
Thermal analysis study is performed using differential scanning calorimeter.
5. Morphological characterization
Morphological characters are studied by using scanning electron microscope (SEM).
6. Water absorption capacity test
Circular Patches, with a surface area of 2.3 cm2 are allowed to swell on the surface of agar plates prepared in simulated saliva (2.38 g Na2HPO4, 0.19 gKH2PO4, and 8 g NaCl per litter of distilled water adjusted withphosphoric acid to pH 6.7), and kept in an incubator maintained at 37°C ± 0.5°C. At various time intervals (0.25, 0.5, 1, 2, 3, and 4 hours), samples are weighed (wet weight) and then left to dry for 7 days in a desiccator over anhydrous calcium chloride at room temperature then the final constant weights are recorded. Water uptake (%) is calculated using the following equation
Water uptake (%) = (Ww-Wf) x 100 / Wf
Where, Ww is the wet weight and Wf is the final weight. The swelling of each film is measured
7. Ex-vivo bioadhesion test
The fresh sheep mouth separated and washed with phosphate buffer (pH 6.8). A piece of gingival mucosa is tied in the open mouth of a glass vial, filled with phosphate buffer (pH 6.8). This glass vial is tightly fitted into a glass beaker filled with phosphate buffer (pH 6.8, 37°C ± 1°C) so it just touched the mucosal surface. The patch is stuck to the lower side of a rubber stopper with cyano acrylate adhesive. Two pans of the balance are balanced with a 5-g weight. The 5-g weight is removed from the left hand side pan, which loaded the pan attached with the patch over the mucosa. The balance is kept in this position for 5 minutes of contact time. The water is added slowly at 100 drops/min to the right-hand side pan until the patch detached from the mucosal surface. The weight, in grams, required to detach the patch from the mucosal surface provided the measure of mucoadhesive strength (Figure 2).
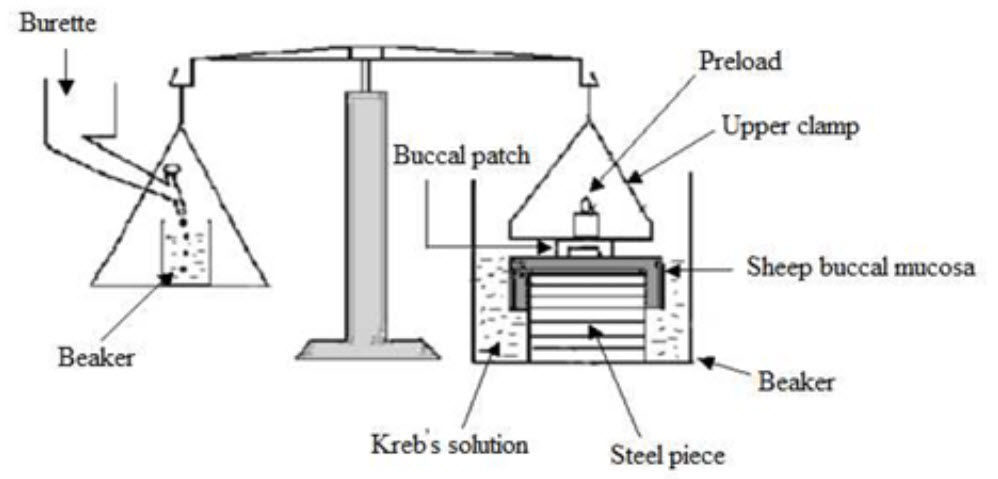
8. In vitro drug release
The United States Pharmacopeia (USP) XXIII-B rotating paddle method is used to study the drug release from the bilayered and multilayered patches. The dissolution medium consisted of phosphate buffer pH 6.8. The release is performed at 37°C ± 0.5°C, with a rotation speed of 50 rpm. The backing layer of buccal patch is attached to the glass disk with instant adhesive material. The disk is allocated to the bottom of the dissolution vessel. Samples (5 ml) are withdrawn at pre determined time intervals and replaced with fresh medium. The samples filtered through whatman filter paper and analyzed for drug content after appropriate dilution. The in- vitro buccal permeation through the buccal mucosa (sheep and rabbit) is performed using Keshary-Chien/Franz type glass diffusion cell at 37°C± 0.2°C. Fresh buccal mucosa is mounted between the donor and receptor compartments. The buccal patch is placed with the core facing the mucosa and the compartments clamped together. The donor compartment is filled with buffer (Figure 3).
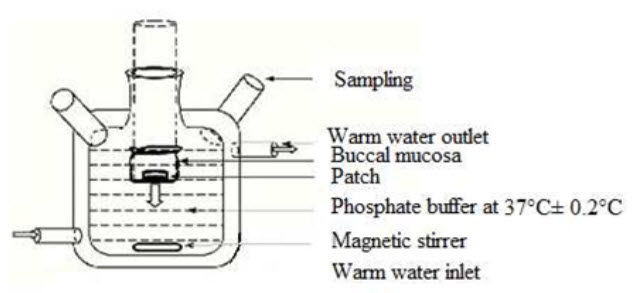
9. Permeation study of buccal patch
The receptor compartment is filled with phosphate buffer pH 6.8, and the hydrodynamics in the receptor compartment is maintained by stirring with a magnetic bead at 50 rpm. Samples are withdrawn at predetermined time intervals and analyzed for drug content.
10. Ex-vivo mucoadhesion time
The ex-vivo mucoadhesion time performed after application of the buccal patch on freshly cut buccal mucosa (sheep and rabbit). The fresh buccal mucosa is tied on the glass slide, and a mucoadhesive patch is wetted with 1 drop of phosphate buffer pH 6.8 and pasted to the buccal mucosa by applying a light force with a fingertip for 30 seconds. The glass slide is then put in the beaker, which is filled with 200 ml of the phosphate buffer pH 6.8, is kept at 37°C ± 1°C. After 2 minutes, a 50-rpm stirring rate is applied to simulate the buccal cavity environment, and patch adhesion is monitored for 12 hours. The time for changes in colour, shape, collapsing of the patch, and drug content is noted.
11. Measurement of mechanical properties
Mechanical properties of the films (patches) include tensile strength and elongation at break is evaluated using a tensile tester. Film strip with the dimensions of 60 x 10 mm and without any visual defects cut and positioned between two clamps separated by a distance of 3 cm. Clamps designed to secure the patch without crushing it during the test, the lower clamp held stationary and the strips are pulled apart by the upper clamp moving at a rate of 2 mm/sec until the strip break. The force and elongation of the film at the point when the trip break is recorded. The tensile strength and elongation at break values are calculated using the formula.
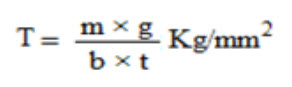
Where,
M - is the mass in gm, g - is the acceleration due to gravity 980 cm/sec2
B - is the breadth of the specimen in cm
T - is the thickness of specimen in cm.
Tensile strength (kg/mm2) is the force at break (kg) per initial cross- sectional area of the specimen (mm2).
CONCLUSION
The buccal mucosa offers several advantages for controlled drug delivery for extended periods of time. The mucosa is well supplied with both vascular and lymphatic drainage and first-pass metabolism in the liver and pre-systemic elimination in the gastrointestinal tract are avoided. The area is well suited for a retentive device and appears to be acceptable to the patient. With the right dosage form design and formulation, the permeability and the local environment of the mucosa can be controlled and manipulated in order to accommodate drug permeation. Buccal drug delivery is a promising area for continued research with the aim of systemic delivery of orally inefficient drugs as well as a feasible and attractive alternative for non-invasive delivery of potent peptide and protein drug molecules. However, the need for safe and effective buccal permeation/absorption enhancers is a crucial component for a prospective future in the area of buccal drug delivery.
REFERENCES
1. Giradkar KP, et al, Design development and in vitro evaluation of bioadhesive dosage form for buccal route, International journal of pharma research & development, 2010,
2. Shidhaye SS, et al, Mucoadhesive bilayered patches for administration of sumatriptan, AAPS pharm sci tech, 2009, 9(3).
3. Amir H, et al, Systemic drug delivery via the buccal mucosal route, Pharmaceutical technology, 2001, 1-27.
4. Pramodkumar TM et al, Oral transmucosal drug delivery systems, Indian drug, 2004, 41(2), 63-12.
5. Edsman K, et al, Pharmaceutical applications of mucoadhesion for the non-oral routes, Journal of pharmacy & pharmacology, 2005, 57, 3-19.
6. Steward A et al, The Effect of Enhancers on the Buccal Absorption of Hybrid (BDBB) Alpha-Interferon, Int.J. Pharm, 104, 1994, 145–149.
7. Aungst BJ and Rogers NJ, Site Dependence of Absorption- Promoting Actions of Laureth9, Na Salicylate, Na2EDTA, and Aprotinin on Rectal, Nasal, and Buccal Insulin Delivery, Pharm. Res, 1988, 5 (5), 305–308
8. Formulation and Evaluation of Buccal Patches of Terbutaline Sulphate Peeush Singhal et al. | Int. J. Res. Pharm. Sci. Vol-1, Issue-4, 440-449, 2010
9.TRANSDERMAL PATCH: A DISCRETE DOSAGE FORM by KAMAL SAROHA International Journal of Current Pharmaceutical Research ISSN- 0975-7066 Vol 3, Issue 3, 2011.
10.THE THEORY AND PRACTICE OF INDUSTRIAL PHARMACY, LEON LACHMAN AND HEBERT A. LIEBERMAN.
11.THE SCIENCE OF DOSAGE FORM DESIGHN BY AULTON.
12.THE SCIENCE AND PRACTICE OF PHARMACYBY REMINGTON.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









