{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Keshari Roshan*, Rathore KS, Bharkatiya Meenakshi, Mishra Amul
Bhupal Nobel’s Institute of Pharmaceutical Sciences,
Udaipur, Rajasthan, India.
*Roshankeshari220@gmail.com
ABSTRACT
Microencapsulation is a process in which a very tiny droplet of particle such as solid, liquid or even gas can be entrapped, coated or surrounded with a polymeric particle. There are different technique to encapsulate the material by chemical method which includes coacervation method, polymeric-polymeric incompatibility, and physical method which include air suspension method, pan coating, spray drying, and centrifugal extrusion. The main important material used in microencapsulation is core material (which is specified material to be coated) and coating material (which is capable of forming film). Since it is applicable in pharma industry, agriculture industry, food industry, construction industry. As it is better drug delivery system than conventional drug delivery system with minimum side effect and having targeted action.
INTRODUCTION
Microencapsulation is a process by which very tiny droplets or particles of liquid, solid or even gas material are surrounded or coated with a continuous film of polymeric material. It includes Bioencapsulation which is more restricted to the entrapment of a biologically active substance (from DNA to entire cell or group of cells for example) generally to improve its performance or enhance its shelf life .
The process had its origin in the late 1930s as a cleaner substitute for carbon paper and carbon ribbons as sought by the business machines industry. The ultimate development in the 1950s of reproduction paper and ribbons that contained dyes in tiny gelatin capsules released on impact by a typewriter key or the pressure of a pen or pencil was the stimulus for the development of a host of microencapsulated materials, including drugs.
The definition has been expanded, and includes more foods. Every class of food ingredient has been encapsulated; flavors are the most common. The technique of microencapsulation depends on the physical and chemical properties of the material to be encapsulated .These micro-capsules have a number of benefits such as converting liquids to solids, separating reactive compounds, providing environmental protection, improved material handling properties. Active materials are then encapsulated in micron-sized capsules of barrier polymers (gelatin, plastic, wax ...).

Figure 1 – Microencapsulation process
Reason for microencapsulation
There are many reasons towards microencapsulation. In some cases, the core must be isolated from its surroundings, as in isolating vitamins from the deteriorating effects of oxygen, retarding evaporation of a volatile core, improving the handling properties of a sticky material or isolating a reactive core from chemical attack. There are several reasons why substances may be encapsulated.
1. To control release of the active components for delayed (timed) release or long-acting (sustained) release.
2. The drugs, which are sensitive to oxygen, moisture or light, can be stabilized by microencapsulation.
3. Incompatibility among the drugs can be prevented by microencapsulation.
4. Vaporization of many volatile drugs e.g. methyl salicylate and peppermint oil can be prevented by microencapsulation.
5. Many drugs have been microencapsulated to reduce toxicity and GI irritation including ferrous sulphate and KCl.
6. Alteration in site of absorption can also be achieved by microencapsulation.
7. Toxic chemicals such as insecticides may be microencapsulated to reduce the possibility of sensitization of factorial person.
8. Bakan and Anderson reported that microencapsulated vitamin A palmitate had enhanced stability [1].
[adsense:468x15:2204050025]
MATERIALS AND METHODS FOR MICROENCAPSULATION
Preparation of microspheres should satisfy certain criteria, like basic understanding of the general properties of microcapsules, such as the nature of the core and coating materials, the stability and release characteristics of the coated materials and the microencapsulating methods.
Core material
The core material, defined as the specific material to be coated, can be liquid or solid in nature. The composition of the core material can be varied as the liquid core can include dispersed and/or dissolved material. The solid core can be mixture of active constituents, stabilizers, diluents, excipients and release-rate retardants or accelerators. The ability to vary the core materials composition provides definite flexibility and utilization of this characteristic often allows effectual design and development of the desired microcapsules properties. Table 1 illustrates core material and its characteristic as well as purpose of the encapsulation.
Table 1 - core material and characteristic
|
Core material |
Characteristic property |
Purpose of encapsulation |
Film product form |
|
Acetaminophen |
Slightly water soluble solid |
Taste masking |
Tablet |
|
Vitamin –A palmitate |
Non volatile liquid |
Stabilization to oxidation |
Dry powder |
|
Activated charcoal |
Adsorbent |
Selective sorption |
Dry powder |
|
Liquid crystal |
Liquid |
Conversion of liquid to solid stabilizer |
Flexible film for thermal mapping for anatomy |
|
Potassium chloride |
Highly water soluble solid |
Reduce gastric irritation |
Capsule |
|
Aspirin |
Slightly water soluble solid |
Taste masking ,sustained release, reduce gastric irritation, separation of incompatibilities |
Tablet or capsule |
|
Urease |
Water-soluble enzyme |
Selectivity of enzyme,substrate,and reaction products |
Dispersion |
|
Islet of Langer Hans |
Viable cells |
Sustained normalization of diabetic condition |
Injectable |
|
Progesterone |
Slightly water soluble solid |
Sustained release |
Varied |
|
Menthol/methyl salicylate camphor mixture |
Volatile solution |
Reduction of volatility; sustained Release |
Lotion |
|
Isosorbide dinitrite |
Water-soluble solid |
Sustained release |
Capsule |
Coating material
The coating material should be capable of forming a film that is cohesive with the core material, chemically compatible and nonreactive with the core material, Stability with core material, Inert toward active ingredients, Controlled release under specific conditions, the coating can be flexible, brittle, hard, thin etc, Abundantly and cheaply available . It also provides the desired coating properties, such as strength, flexibility, impermeability, optical properties, and stability. The coating materials used in microencapsulation methods are amenable, to some extent, to in situ modification.
The selection of a given coating often can be aided by the review of existing literature and by the study of free or cast films, although practical use of free-film information often is impeded for the following reasons:
1. Cast or free films prepared by the usual casting techniques yield films that are considerably thicker than those produced by the microencapsulation of small particles, hence the results obtained from the cast films may not be extrapolate to the thin microcapsule coatings.
2. The particular microencapsulation method employed for the deposition of a given coating produces specific and inherent properties that are difficult to simulate with existing film-casting methods.
3. The coating substrate of core material may have a decisive effect on coating properties. Hence, the selection of a particular coating material involves consideration of both classic free-film data and applied result.
Composition of coating
• Inert polymer
• Plasticizer
• Colouring agent
Microencapsulation technique
There numerous technologieshas been available for the encapsulation of core material have been reported [2, 3, and 4]. These different microencapsulation techniques are more relevant to the coating industries and also provide a comprehensive review of recently developed methods. In general, microencapsulation techniques are divided into two basic groups, namely chemical and physical, with the latter being further subdivided into physico-chemical and physico-mechanical techniques. Some of the important processes used for microencapsulation are summarized in the table -2
Different techniques used for microencapsulation along with their particle size range
|
Technique |
Methods used |
Particle size range [μm] |
|
Coacervation |
PHYSICO – CHEMICAL |
2 – 1200 |
|
Polymer-polymer incompatibility |
PHYSICO – CHEMICAL |
0.5 – 1000 |
|
Encapsulation by supercritical Fluid Encapsulation by Polyelectrolyte multilayer |
PHYSICO - CHEMICAL
|
0.02 – 20 |
|
Phase Inversion |
PHYSICO – CHEMICAL |
0.5—5.0 |
|
Hot Melt |
PHYSICO – CHEMICAL |
1—1000 |
|
Spray-drying |
PHYSICO – MECHANICAL |
5 – 5000 |
|
Fluidized- bed technology |
PHYSICO – MECHANICAL |
20 – 1500 |
|
Pan coating |
PHYSICO – MECHANICAL |
600 – 5000 |
|
Spinning disc |
PHYSICO – MECHANICAL |
5 – 1500 |
|
Co-extrusion |
PHYSICO – MECHANICAL |
250 – 2500 |
|
Interfacial polymerization |
PHYSICO – MECHANICAL |
0.5 – 1000 |
|
In situ polymerization (0.5 – 1100 um) |
PHYSICO – MECHANICAL |
0.5 – 1100 |
|
Layer-by-layer (LBL) assembly |
PHYSICO - CHEMICAL |
0.02–20 |
|
Sol-gel encapsulation |
PHYSICO - CHEMICAL |
2–20 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHYSIO-CHEMICAL PROCESS
coacervation
The first systematic approach of phase separation – that is, partial desolvation of a homogeneous polymer solution into a polymer-rich phase (coacervate) and the poor polymer phase (coacervation medium) – was realized by Bungenberg and colleagues [5,6]. These authors termed such a phase separation phenomenon “coacervation”. The term originated from the Latin ›acervus‹, meaning “heap”. This was the first reported process to be adapted for the industrial production of microcapsules.
It is generally attributed to The National Cash Register (NCR) Corporation and the patents of B.K. Green et al. The process consists of three steps [7].
Two methods for coacervation are available, namely simple and complex processes.
• Simple coacervation involves a desolvation agent is added for phase separation.
• Complex coacervation involves complexation between two oppositely charged polymers in a solvent usually water.
The three basic steps in complex coacervation are
•Preparation of the dispersion or emulsion
• Encapsulation of the core
• Stabilization of the encapsulated particle
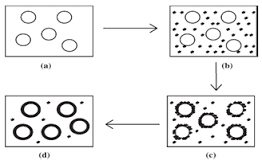
Figure-2 schematic representation of the coacervation process. (A) core material dispersion in solution of polymer shell; (B) separation of coacervate from solution; (c)coating of core material by micro droplet of coacervate (D) coalescence of coacervate to form continuous shell around core particle.
polymer-polymer incompatibility
This method utilizes two polymers that are soluble in a common solvent; yet do not mix with one another in the solution. The polymers form two separate phases, one rich in the polymer intended to form the capsule walls, the other rich in the incompatible polymer meant to induce separation of the two phases. The second polymer is not intended to be part of the finished microcapsule wall.
Fluid Encapsulation by Polyelectrolyte multilayer
Microencapsulation has also been carried out by rapid expansion of supercritical fluid [8,9]. Supercritical fluids are highly compressed gases that possess several advantageous properties of both liquids and gases. Most widely used ones are supercritical CO2, alkanes (C2 to C4) and nitrous oxide (N2O). Supercritical CO2 is widely used for its low critical temperature value in addition to its non-toxic and non-flammable properties. It is also readily available, highly pure and cost effective. It has found applications in encapsulating active ingredients by polymers. Different core materials such as pesticides, pigments, pharmaceutical ingredients, vitamins, flavors and dyes have been encapsulated by using this method. A wide variety of shell materials that either dissolve paraffin wax, acrylates, polyethylene glycol or do not dissolve proteins, polysaccharides in supercritical CO2 are used for encapsulating core substances. In this process, supercritical fluid containing the active ingredient and the shell material are maintained at high pressure and then released at atmospheric pressure through a small nozzle. The sudden drop in pressure causes desolvation of the shell material, which is then deposited around the active ingredient (core) and forms a coating layer. Felodipine has been encapsulated in polyethylene glycol by using this Technique [10].
The most widely used methods are as follows:
●Rapid expansion of supercritical solution (RESS)
●Gas anti-solvent (GAS)
●Particles from gas-saturated solution (PGSS)
Process Involved
●Supercritical fluid contains the active ingredient and the shell material are maintained at high pressure and then released at atmospheric pressure through a small nozzle.
●The sudden drop in pressure causes desolvation of the shell material, which is then deposited around the active ingredient (core) and forms a coating layer.
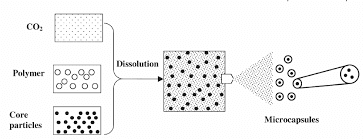
Figure-3 Microencapsulation by rapid expansion of supercritical solutions
PHYSIO-MECHANICAL METHOD
Air suspension coating-
Air-suspension coating, first described by Professor Dale Erwin Wurster at the University of Wisconsin in 1959, [11]gives improved control and flexibility compared to pan coating. In this process the particulate core material, which is solid, is dispersed into the supporting air stream and these suspended particles are coated with polymers in a volatile solvent leaving a very thin layer of polymer on them. This process is repeated several hundred times until the required parameters such as coating thickness, etc., are achieved. The air stream which supports the particles also helps to dry them, and the rate of drying is directly proportional to the temperature of the air stream which can be modified to further affect the properties of the coating.
The re-circulation of the particles in the coating zone portion is effected by the design of the chamber and its operating parameters. The coating chamber is arranged such that the particles pass upwards through the coating zone, then disperse into slower moving air and sink back to the base of the coating chamber, making repeated passes through the coating zone until the desired thickness of coating is achieved.
Pan coating
The pan coating process, widely used in the pharmaceutical industry, is among the oldest industrial procedures for forming small, coated particles or tablets. The particles are tumbled in a pan or other device while the coating material is applied slowly[ 12] with respect to microencapsulation, solid particles greater than 600 microns in size are generally considered essential for effective coating, and the process has been extensively employed for the preparation of controlled - release beads. Medicaments are usually coated onto various spherical substrates such as nonpareil sugar seeds, and then coated with protective layers of various polymers .
In this method, solid particles are mixed with a dry coating material and the temperature is raised so that the coating material melts and encloses the core particles, and then is solidified by cooling; or, the coating material can be gradually applied to core particles tumbling in a vessel rather than being wholly mixed with the core particles from the start of encapsulation.
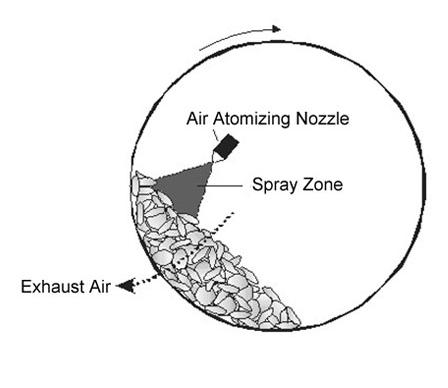
Figure-4 Representation of typical pan coating
Centrifugal extrusion
This process possesses a number of commercial applications. Centrifugal extrusion processes generally produce capsules of a larger size, from 250 microns up to a few millimeters in diameter. The core and the shell materials, which should be immiscible with one another, are pushed through a spinning two-fluid nozzle. This movement forms an unbroken rope which naturally splits into round droplets directly after clearing the nozzle. The continuous walls of these droplets are solidified either by cooling or by a gelling bath, or chemical cross linking depending on the composition and properties of the coating material. Hepatocytes have been encapsulated in polyacrylonitrile77 by using this technique.
This process is excellent for forming particles 400–2,000 µm (16–79 mils) in diameter. Since the drops are formed by the breakup of a liquid jet, the process is only suitable for liquid or slurries. A high production rate can be achieved, up to 22.5 kg (50 lb) of microcapsules can be produced per nozzle per hour. Heads containing 16 nozzles are available.
spray drying
Microencapsulation by spray-drying is a low-cost commercial process which is mostly used for the encapsulation of fragrances, oils and flavors. Spray drying is the continuous transformation of feed from a fluid state into dried particulate form by spraying the feed into a hot drying medium. An emulsion is prepared by dispersing the core material, usually an oil or active ingredient immiscible with water; into a concentrated solution of wall material until the desired size of oil droplets are attained. The resultant emulsion is atomized into a spray of droplets by pumping the slurry through a rotating disc into the heated compartment of a spray drier. There the water portion of the emulsion is evaporated, yielding dried capsules of variable shape containing scattered drops of core material. The capsules are collected through continuous discharge from the spray drying chamber[13].This method can also be used to dry small microencapsulated materials from aqueous slurry that are produced by chemical methods. Lycopene has been microencapsulated inside gelatin microcapsules by using this technique70.
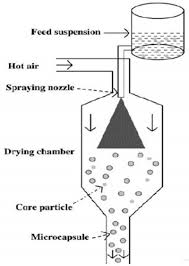
Figure -5 Schematic illustrating the process of micro-encapsulation by spray-drying.
APPLICATIONS
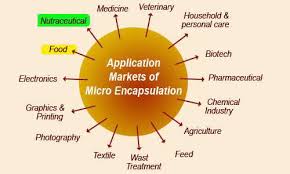
Figure-6 application of microencapsulation
In industrial applications, the objective is not only to isolate the core completely but to control the rate at which it leaves the microcapsule, as in the controlled release of citric acid in the food industry and chemical drugs in the pharma industry and fertilizers in the agro industry. Actually about any area in the industry could beneficiate from microencapsulation technologies.
Agriculture
Microencapsulation can be found in various fields One of the most important applications of microencapsulated products is in the area of crop protection . Nowadays insect pheromones are becoming viable as a biorational alternative to conventional hard pesticides. Specifically, sex attractant pheromones can reduce insect populations by disrupting their mating process. Hence small amounts of species- specific pheromone are dispersed during the mating season, raising the background level of pheromone to the point where it hides the pheromone plume released by its female mate. Polymer microcapsules, polyurea gelatin and gum Arabic serve as efficient delivery vehicles to deliver the pheromone by spraying the capsule dispersion. Further, encapsulation protects the pheromone from oxidation and light during storage and release.
Pharmaceutics
One of the major applications area of encapsulation technique is pharmaceutical/ biomedical for controlled/sustained drug delivery. Potential applications of this drug delivery system are replacement of therapeutic agents (not taken orally today like insulin), gene therapy and in use of vaccines for treating AIDS, tumors cancer and diabetes. Protein such as insulin, growth hormone, and erythropoietin (used to treat anemia) are example of drugs that would benefit from this new form of oral delivery.
Based on this novel drug delivery technique, Lupin has already launched in the market world’s first Cephalexin (Ceff-ER) and Cefadroxil (Odoxil OD) antibiotic tablets for treatment of bacterial infections. Aspirin controlled release version ZORprin CR tablets are used for relieving arthritis symptoms. Quinidine gluconate CR tablets are used for treating and preventing abnormal heart rhythms. Niaspan CR tablet is used for improving cholesterol levels and thus reducing the risk for a heart attack. Glucotrol (Glipizide SR) is an anti diabetic medicine used to control high blood pressure.
Cell Immobilization:
In plant cell cultures microencapsulation, by mimicking cell natural environment, improves efficiency in production of different metabolites used for medical, pharmacological and cosmetic purposes. Human tissue are turned into bioartificial organs by encapsulation in natural polymers and transplanted to control hormone deficient diseases such as diabetes and severe cases of hepatic failure. In continuous fermentation processes immobilization is used to increase cell density, productivity and to avoid washout of the biological catalysts from the reactor. This has already been applied in ethanol and solvent production, sugar conversion or wastewater treatment.
Beverage Production:
Today beer, wine, vinegar and other food drinks production are using immobilization technologies to boost yield, improve quality, change aromas, etc.
Applications of microcapsules in building construction materials
An analysis of scientific articles and patents shows numerous possibilities of adding microencapsulated active ingredients into construction materials, such as cement, lime, concrete, mortar, artificial marble, sealants, paints and other coatings, and functionalized textiles.
Energy Generation
Hollow plastic microspheres loaded with gaseous deuterium (a fusion fuel) are used to harness nuclear fusion for producing electrical energy. The capsules are multilayered. The inner layer, which compresses the fuel, is a polystyrene shell about 3 mm thick. Next is a layer of poly(vinyl alcohol) about 3 mm thick, that retards diffusion of deuterium out of the capsule. The outer layer (the ablator) is about 50 mm thick and consists of a highly cross linked polymer made from 2-butene. During the fusion experiments, energy from high powered laser beams is absorbed by the surface of the microcapsule shell. As the outside of the shell (called ablator) burns off, the reaction force accelerates the rest of the shell inward, compressing and heating the deuterium inside. These result in high densities and temperature in the centre of the capsule leading to the fusion of deuterium nuclei to give tritium, helium and other particles releasing an enormous amount of energy. This process has been named as inertial confinement fusion (ICF). Such ICF targets made of organic microcapsules have been in use since 1980s.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHYSIOLOGICAL EVALUATION AND CHARACTERIZATION
ANGLE OF CONTACT
The angle of contact is measured to determine the wetting property of a micro particulate carrier. It determines the nature of microspheres in terms of hydrophilicity or hydrophobicity. This thermodynamic property is specific to solid and affected by the presence of the adsorbed component. The angle of contact is measured at the solid/air/water interface. The advancing and receding angle of contact are measured by placing a droplet in a circular cell mounted above objective of inverted microscope. Contact angle is measured at 200C within a minute of deposition of microspheres [14].
SIEVE ANALYSIS
Separation of the microspheres into various size fractions can be determined by using a mechanical sieve shaker (Sieving machine, Retsch, Germany). A series of five standard stainless steel sieves (20, 30, 45, 60 and 80 mesh) are arranged in the order of decreasing aperture size. Five grams of drug loaded microspheres are placed on the upper-most sieve. The sieves are shaken for a period of about 10 min, and then the particles on the screen are weighed [15].
VISCOSITY OF THE POLYMER SOLUTIONS
The absolute viscosity, kinematic viscosity, and the intrinsic viscosity of the polymer solutions in different solvents can be measured by a U-tube viscometer (viscometer constant at 40 0C is 0.0038 mm2/s /s) at 25 ± 0.1 0C in a thermostatic bath. The polymer solutions are allowed to stand for 24 h prior to measurement to ensure complete polymer dissolution [16].
MORPHOLOGY OF MICROSPHERES
The surface morphologies of microspheres are examined by a scanning electron microscope (XL 30 SEM Philips, Eindhoven, and The Netherlands). The microspheres are mounted onto a copper cylinder (10 mm in diameter, 10 mm in height) by using a double-sided adhesive tape. The specimens are coated at a current of 10 mA for 4 min using an ion sputtering device (JFC-1100E, Jeol, Japan) [15, 16].
BULK DENSITY
The microspheres fabricated are weighed and transferred to a 10-ml glass graduated cylinder. The cylinder is tapped using an autotrap (Quantach- rome, FL, USA) until the microsphere bed volume is stabilised. The bulk density is estimated by the ratio of microsphere weight to the final volume of the tapped microsphere bed [17].
ATOMIC FORCE MICROSCOPY (AFM)
A Multimode Atomic Force Microscope from Digital Instrument is used to study the surface morphology of the microspheres. The samples are mounted on metal slabs using double-sided adhesive tapes and observed under microscope that is maintained in a constant-temperature and vibration-free environment [18].
PARTICLE SIZE
Particle size determination approximately 30 mg microparticles is redispersed in 2–3 ml distilled water, containing 0.1% (m /m) Tween 20 for 3 min, using ultrasound and then transferred into the small volume recirculating unit, operating at 60 ml/ s. The microparticle size can be determined by laser diffractometry using a Malvern Mastersizer X (Malvern Instruments, UK).
DENSITY DETERMINATION
The density of the microspheres can be measured by using a multi volume pychnometer. Accurately weighed sample in a cup is placed into the multi volume pychnometer. Helium is introduced at a constant pressure in the chamber and allowed to expand. This expansion results in a decrease in pressure within the chamber. Two consecutive readings of reduction in pressure at different initial pressure are noted. From two pressure readings the volume and density of the microsphere carrier is determined.
STATUS OF MICROENCAPSULATION RESEARCH IN DMSRDE
Defence Materials and Stores Research and Development Establishment, Kanpur is actively working in this area since last four years and has developed expertise in microencapsulation technology by using two techniques. One is solvent evaporation and the other one is interfacial polymerisation. Characterisation of all the synthesized microcapsules was done by using optical microscope (OM, Leica) and scanning electron microscope (SEM, Zeiss).
Polymethylmethacrylate (PMMA) was selected as the polymer for encapsulation using solvent evaporation technique. Different materials like carbon nanotubes (CNTs), polyaniline, carbon microcoils (CMCs) and magnetic nanoparticles have been successfully encapsulated inside PMMA microcapsules. In a typical synthesis for encapsulation of polyaniline, PMMA microcapsules. The formation of microcapsule is clearly evident from the micrograph. A perfect sphere is seen with the presence of green polyaniline inside the sphere. Using the same procedure microencapsulation of other materials was also carried out.
FUTURE TRENDS OF MICROENCAPSULATION
Microencapsulation is not new. It has been around for decades in the form of spray drying, spray chilling, freeze drying and coacervation. But scientists believe that the sector has innovated rapidly. The microencapsulation sector is therefore fast establishing itself at the cutting edge of food and beverage flavor development. The use of nanotechnology, which involves the study and use of materials at sizes of millionths of a millimeter, could increasingly be used in the creation and development of flavors and flavor systems in the future. Future prospects of microencapsulation of islets of Langerhans used sodium alginate and poly-l-lysine (PLL) to form the capsules.
CONCLUSION
In Microencapsulation process solid, liquid and even gas can be entrapped. And has got more popularity in drug delivery system and has been shown most advantageous than conventional drug delivery system. Since it is not only used in pharmaceutical field only but it is also used in agricultural field, analysis field in food industry as well as in building construction material also. As there are many method of encapsulation but still it is on research to develop more method to encapsulate different matter and better than previous method.
Acknowledgment
Very first I respectfully acknowledge this work to my Parents [NandKishor Prasad Keshari (Father) and Babita Devi Keshari (Mother)], and Family Members and teachers, who made me genius in field of education. It is said that accomplishments must be credited to those who have put up the foundations of the particular chore: here I pay tributes to my parents for lifting me up till this phase of life. I am also thankful to my dearest brother Suraj, Dheeraj and lovely sister Shikha, and my most helpful friend Dia erohtar for their encouragement, love and support which have boosted me morale. Thanking you all.
REFERENCES
1. James, S., “Encylopedia of Pharmaceutical Techonology”, 3rd edition, Vol-, 1325-1333.
2. S. Benita, Microencapsulation: Methods and Industrial applications, Marcel Dekker, Inc., New York, 1996.
3. R. Arshady, Microspheres, Microcapsules and Liposomes, Citrus Books, London, United Kingdom, 1999.
4. M.W. Ranney, Microencapsulation Technology, Noyes Development Corporation, Park Ridge, 1969, p. 275.
5. G. Bungenberg de Jong, H. Kruyt, Prog. Kungl. Ned. Acad. Wetensch. 1929, 32, 849–856.
6. H.G. Bungenberg de Jong, in: Colloid Science (Ed. H.R. Kruyt), Elsevier, 1949, pp. 232–258.
7. O’Donnell PB, McGinity JW, Preparation of microspheres by solvent evaporation technique. Advanced Drug Delivery Reviews. 1997;28:25-42.
8. Chiou, A. H.; Cheng, H. C. & Wang, D. P. Micronization and microencapsulation of felodipine by supercritical CO2. J. Microencap., 2006, 23(3) 265-76.
9. Cochrum, K. C. & Davis The reguents of the university of California, Oakland, California. Spin disk encapsulation apparatus and method of use. US Patent 6,001,387. 14 Dec 1999. 22pp.
10. Jackson, L. S., Lee., K., (1991-01-01), “ Microencapsulation and the food industry ”(htm) Lebennsmittel- Wissenschaft Techonologie. Rerrived on 1991-02-02.
11. Desai, K. G. H.1 Park, H. J.2, Preparation of cross-linked chitosan microspheres by spray drying: Effect of cross-linking agenton the properties of spray dried microspheres, Journal of Microencapsulation: 22(4), June, p. 377- 395(19),( 2005).
12. Alagusundaram.M, Madhu Sudana chetty, C.Umashankari. Microspheres as a Novel drug delivery system – A review. International J of chem. Tech.2009: 526-534.
13. Fang-Jing Wang, Chi-Hwa Wang. Sustained release of etanidazole from spray dried microspheres prepared by nonhalogenated solvents. Journal of Controlled Release 81 (2002) 263–280
14. Scher, H. B.; Rodson, M & Lee, K. S. Microencapsulation of pesticides by interfacial polymerization utilizing isocyanate or aminoplast chemistry. Pestic. Sci., 1998, 54, 394-400.
15. Bingham, G.; Gunning, R.V.; Gorman, K.; Field, L.M. & Moores, G.D. Temporal synergism by microencapsulation of piperonyl butoxide and cypermethrin overcomes insecticide resistance in crop pests. Pest Mgmt. Science, 2007, 63, 276-81.
16. Ilichev, A.L.; Stelinski, L.L.; Williams, D.G. & Gut, L.J. Sprayable microencapsulated sex pheromone formulation for mating disruption of oriental fruit moth (Lepidoptera: Tortricidae) in Australian peach and pear orchards. J. Econ. Entomol., 2006, 99(6), 2048-054.
17. Mihou, A. P. ; Michaelakis, A. ; Krokos, F. D.; Majomenos, B. E. & Couladouros, E. A. Prolonged slow release of (Z)-11-hexadecenyl acetate employing polyurea microcapsules. J. Appl. Entomol., 2007, 131(2), 128-33.
18. Zengliang, C.; Yuling, F. & Zhongning, Z. Synthesis and assessment of attractiveness and mating disruption efficacy of sex pheromone microcapsules for the diamondback moth, Plutella Xylostella (L). Chinese Sci. Bull., 2007, 57(10), 1365-71.
REFERENCE ID: PHARMATUTOR-ART-2450
|
PharmaTutor (ISSN: 2347 - 7881) Volume 4, Issue 12 Received On: 01/07/2016; Accepted On: 25/07/2016; Published On: 01/12/2016 How to cite this article: Keshari R, Rathore KS, Bharkatiya M, Mishra A; Microencapsulation drug delivery system - an overview; PharmaTutor; 2016; 4(12); 20-28 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









