About Authors:
Ramit Singla1, Arvind Negi1*, Virendra Singh2
1Centre for Chemical and pharmaceutical sciences, Central university of Punjab, Bathinda, India
2Department of Chemistry, National institute of technology, Jalandhar, India
*arvindnegi2301@gmail.com
{ DOWNLOAD AS PDF }
Abstract:
Indole alkaloids are well characterized for diverse activities. Meanwhile, the present scenario of disease depicting a different story, urge a call for novel scaffolds and pharmacophores. In recent years, indole based alkaloids gain significant importance in cancer chemotherapy and used as an adjuvant especially in case of Vinca alkaloids. These reports encourage the researchers for new finding related to indole based alkaloids to retrieve better scaffold for designing and synthesis of anticancer agents. However we tried to compile the literature which cover the recent reports and updates of indole based alkaloids targeting in cancer. Moreover this review paper brings new dimension and interface between the indole based alkaloids and their valuable commitment towards cancer targeting.
REFERENCE ID: PHARMATUTOR-ART-2089
Introduction
Cancer cells are quite similar to the normal cells, expect the advancement in the signalling which makes them immortal. They readily adapt to starvation microenvironments, and modify their protein-nucleic acid machinery to assure their continued survival so that they can able to utilize the altered mechanisms to promote cellular immortality. All these hallmarks of cancer make challenging task to target the cancerous cells.
Moreover modern cancer chemotherapy utilizes following strategies to target the cancer as follows:
· Combined modality chemotherapy which uses the drugs together with radiation therapy or surgery.
· Neoadjuvant chemotherapy(preoperative treatment)
· Adjuvant chemotherapy(postoperative treatment) is used where there is a risk of recurrence after the surgery.
· Palliative chemotherapyis implicated to increase the life expectancy.
These chemotherapeutic drugs can be majorly classified as:
- Alkylating agents
- Antimetabolites
- Anthracyclines
- Plant alkaloids
- Topoisomerase inhibitors
Natural products, especially those ones which are extracted\obtained from plant origin, have been used for the treatment of various diseases since ancient times and can be witnessed in the numerous ancient\traditional medicinal plant books. In present circumstances where disease modulated themselves to a new level of adaptation (resistance) and modifies.Therefore new scaffolds or pharmacophore are much needed to overcome these issues. Moreover cancer is one such disease and therefore alternatively plant derived agents are being employed for the treatment of cancer. Several natural derived anticancer agents including Taxol, Vinblastine, Vincristine, Camptothecin derivatives, Topotecan and Irinotecan, and Etoposide are in clinical use. Even a number of promising agents such as flavopiridol, roscovitine, combretastatin A-4, betulinic acid and silvestrol are in clinical or preclinical development.
The Indole alkaloids are the major classof alkaloids which turn outinto different anti-cancerous scaffolds, enlisted as:
- Non-isoprenoidtryptamine
- Piperazinedione
- Indoloiminoquinolines
- β-Carbolines
- Bisindole
- Cytochalasins
- Elliptinium
- Vinblastine and vincristine
Indole is a basic heterocyclic scaffold which was originated with the study of a dye, indigo.This scaffold is composed of abenzopyrrole, in which the benzene and pyrrole rings are fusedviaC2 and C3 positions of the pyrrolenucleus(see in Fig.1.).Its presence in the nature is highly versatile even it isfound endogenously in human e.g. tryptophan amino acid. Moreover natural secondary metabolite (alkaloids)contain indole nucleus which is ubiquitously present in nature including plants like (vincristine), marine creatures (manzamines), insects, fungal metabolites (cytochalasins), mammalian as well as human tissues, and body fluids.
Fig. 1. Structure of indole.
This review covers some of the active indole derivatives which have shown anticancer activity. These includes Non-isoprenoid tryptamine, Piperazinedione, Indoloiminoquinolines, β-Carbolines,Bisindole, Cytochalasins, Elliptinium, Vinblastine and Vincristine.
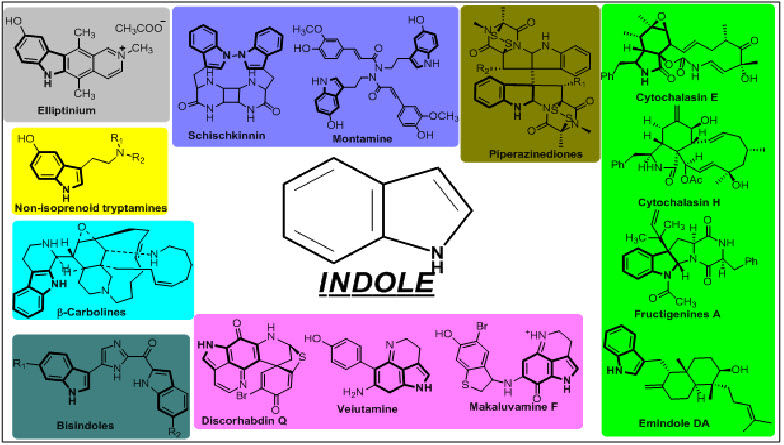
Fig.2. Depicting the different alkaloids possessing the anticancer activity.
Indole-based natural products as Anticancer Agents
Non-isoprenoidtryptamines
Two new bioactive indole alkaloids, Bufobutanoic acid (2) and Bufopyramide (3) wereisolated from the skin secretions of the local toads Bufobufogarigarizans or Bufomelanostrictus. Both compounds exhibited cytotoxicity against murine P388 lymphocytic leukemia cells with IC50 values of 22 µg mL-1 and 26 µg mL-1, respectively[1-4]see in fig.3.
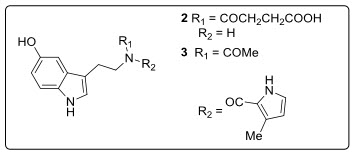
Fig. 3.Structure of anti-cancerous Non-isoprenoid tryptamines.
Piperazinediones
Two cytotoxic epidithiodioxopiperadine dimers, 11,11’-dideoxyverticillin A (4) and 11’ deoxyverticillin A (5) together with known Verticillin A, had been isolated from the mycelium of a marine-derived fungus of the genusPenicillium. Cytotoxicity against HCT-116 human colon carcinoma of compounds (4) and (5) have been described (IC50 = 30 ngML-1)[4]see in fig.4.
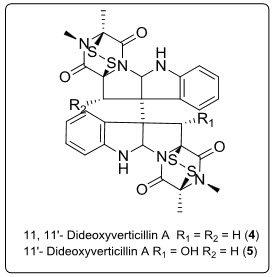
Fig. 4.Structure of piperazinediones alkaloids
Indoloiminoquinolines
A new discorhabdin, Discorhabdin Q (6) (16,17-dehydro-discorhabdin B), had been isolated from cytotoxic extracts of the sponge Latrunculiapurpurea and Zyzzyamassalis, Z. fuligi-nosa, and Z. spp. Discorhabdin Q (6) exhibited moderate cytotoxic activity (GI50 = 0.5 µg ML-1) possessing essentially a differential cytotoxic profile in a NCI 60 cell line antitumor screen[4]see in fig.5.
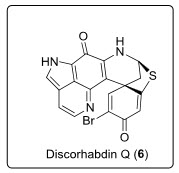
Fig. 5. Structure of anti-cancerous indoloiminoquinolines (Discorhabdin Q).
The first complete synthesis of pyrrolo-iminoquinone marine alkaloid Veiutamine (7), isolated from the Fijian sponge Zyzzya fuliginosa, has been done (Scheme.1.). This alkaloid has potent anticancer activity against solid tumours versus leukaemia (IC50 = 0.12 µg mL-1 in a 25 cell line panel and IC50 = 0.3 µg mL-1 against the human colon tumor cell line HCT-116).
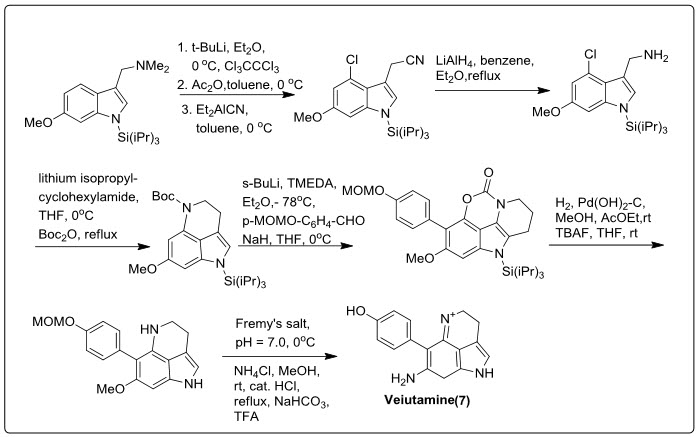
Scheme.1. Synthetic scheme of Indoloiminoquinolines derivative (Veiutamine).
A total synthesis of the marine alkaloid makaluvamine F (8) (see in fig.6.), isolated from the Fijian sponge Zyzzya cf. marsailis and the Indonesian sponge Histodermella sp. (G), has been completed. Makaluvamine F (8) exhibits the potent cytotoxicity towards the human colon tumor cell line HCT-116 (IC50 = 0.17 µM) andthe inhibition of topoisomerase II.
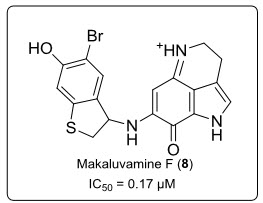
Fig. 6. Structure of Indoloiminoquinolines (Makaluvamine F).
β-Carbolines
A manzaminecongenor, 1,2,3,4-tetrahydro-manzamine B (9) has been isolated from a Okinawan marine sponge Amphimedonsp.Further, its absolute stereochemistry of (9) has been established as (1S)-1,2,3,4-tetrahydromanzamine B (1) by circular dichroism spectroscopy. This compound (9) exhibits cytotoxicity against L1210 murine leukaemia cells and KB human epidermoid carcinoma cells (IC50 = 0.3 and 1.2 µg mL-1, respectively)[5](see in fig.7.).
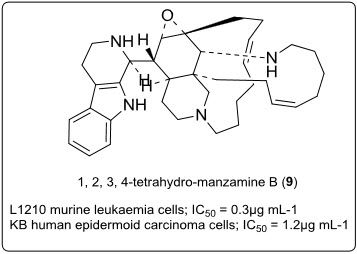
Fig.7.Structure of manzamine cogenor.
Bisindole
Two bisindole alkaloids Bromodeoxytopsentin (10) and Isobromodeoxytopsentin(11) isolated from the sponge Spongosoritesgenitrix together with known deoxy-topsentin (12) and bromotopsentin (13) These compounds displayed moderate cytotoxicity against human leukemia cell-line K-562 (IC50= 0.6 and 2.1 µg ML-1, for (10) and (11), respectively).
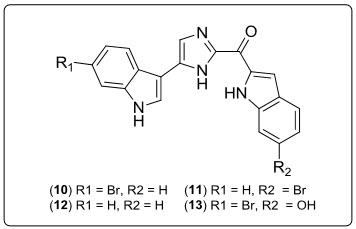
Fig.8.Structure of Bisindole alkaloid derivatives.
Cytochalasins
Fungal endophytes comprises of about 60% of alkaloids with known anti-tumour activity. Among these cytochalasins are interesting because of their broad spectrum anti-tumour activity. Among various metabolites obtained from fungi Rhinocladiella residing on plant Tripterygiumwilfordii,cytochalasin E (14) (see in fig.9.) shown significant activity against three human tumour cell lines 2780S(IC100?0.0153µg/mL),SW-620(colon tumour cell lines IC100=0.244µg/mL) and HCT-116(IC100=0.977µg/mL) respectively.
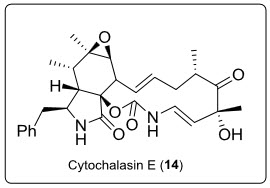
Fig.9. Structure of Cytochalasin.
Cytochalasin H (15) (see in fig.10.), another metabolite isolated from mangrove fungal endophyte Phomopsissp. (ZZF08) in South China Sea coast. It has shown potent cytotoxicity against towards KB and KBv 200 cells with IC50 less than 1.25µg/mL.
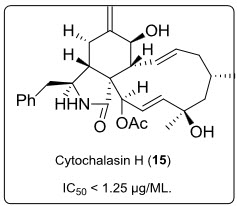
Fig.10. Structrue of Cytochalasin.
Fructigenines A (16) (see in fig.11.), a diketopiperazine alkaloid separated from the endophyticPenicilliumauratiogriseum derived from sponge Mycale plumose by bioassay oriented fractionation showed potent cytotoxic activity against tsFT210 (mouse cdc2 mutant cells) with maximum inhibitory effect at 22µg/mL.
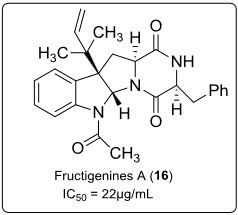
Fig.11.Structure of Fructigenines A.
EmindoleDA (17) (see in fig.12.) an indole alkaloid derived from marine fungus Emericellanidulans var. acristata(a green alga of Mediterranean sea around Sardinia).This alkaloid had exhibited anti-tumour activity on a panel of 36 human tumour cell lines, with IC50= 5.5µg/mL in vitro survival and proliferation assay.
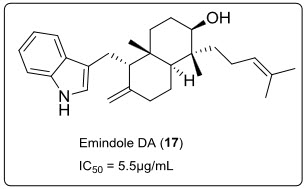
Fig.12.Structure of Emidole.
Elliptinium
A derivative of ellipticine (18) (see in fig.13.), isolated from a Fijian medicinal plant BleekeriavitensisA.C. Sm., is marketed in France for the treatment of breast cancer.
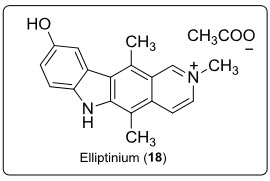
Fig.13. Structure of elliptinum.
Vinblastine and vincristine
Vinblastine and vincristine are alkaloids isolated from Periwinkle.Their solution route of administered majorly viaintravenously in sulphate form. Although if their solutions can be fatal if administered by any other route and can also cause anallergic reactionsor irritation if they leak out of the blood vessels.Vinca alkaloids are useful in the treatment of both malignant and non-malignant diseases (especially in hodgkins lymphoma)[6].
The major mechanism of cytotoxicity of all the vinca alkaloids state to their relations with tubulin and disruption of microtubule function. It leads to metaphasic arrest. They are also able to effect many other accessory downstream intracellular signalling proteins. Also they possess broad spectrum anticancer activityvia-inhibiting synthesis of proteins and nucleic acids, elevating oxidized glutathione, altering lipid metabolism and membrane lipids, elevating cyclic adenosine monophosphate (cAMP), and inhibiting calcium-calmodulin-regulated cAMP phosphodiesterase[7, 8].
Schischkinnin and Montamine
Two novel alkaloids, schischkinnin (19) and montamine (20) (see in fig.14.) were isolated from the seeds of Centaureaschischkinii and Centaureamontana. Both of these alkaloids have shown significant cytotoxicity against human colon cancer cell lines[9, 10].
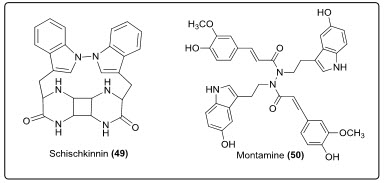
Fig.14.Structure of Schischkinnin and Montamine.
Conclusion:
Recent reports disclosed numerous naturally occurring indole alkaloids were isolated and evaluated against different cancer cell lines where they revealed commendable\appreciable anticancer activity. Therefore it is evident about their potential, to overcome the concerning issues related to the present cancer chemotherapy. The indole scaffold is so versatile that it has shown diverse activities against the different targets in cancer. This special attribute of indole alkaloids can be utilized in rational drug design against the cancer in the near future.
Acknowledgment
I would like to give my special thanks to Dr. Raj Kumar for his support. Along with, I would like to thank Navgeet and all the other persons who supported me during the preparation of this manuscript.
References
1. Somei M, Yamada F: Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Natural product reports2005, 22 (1): 73-103.
2. Toyota M, Ihara M: Recent progress in the chemistry of non-monoterpenoid indole alkaloids. Natural product reports1998, 15 (4): 327-340.
3. Ihara M, Fukumoto K: Recent progress in the chemistry of non-monoterpenoid indole alkaloids. Natural product reports1995, 12 (3): 277-301.
4. Hibino S, Choshi T: Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Natural product reports2001, 18 (1): 66-87.
5. Hibino S, Choshi T: Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Natural product reports2002, 19 (2): 148-180.
6. DeVita VT, SERPICK AA, CARBONE PP: Combination chemotherapy in the treatment of advanced Hodgkin's disease. Annals of Internal Medicine1970, 73 (6): 881-895.
7. Jordan MA, Thrower D, Wilson L: Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer research1991, 51 (8): 2212-2222.
8. Jordan MA, Wilson L: Microtubules as a target for anticancer drugs. Nature Reviews Cancer2004, 4 (4): 253-265.
9. Karim AK, Asmara W: Cytotoxic Activity of Tegari (Dianella nemorosa Lam.) Methanol Extract Against HeLa Cells. Indonesian Journal of Biotechnology2012, 17 (1).
10. Rani P, Kainsa S, Kumar P: Medicinal plants of Asian origin having anticancer potential: short review. Asian Journal of Biomedical and Pharmaceutical Sciences2012, 2 (10): 1-7.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 01/012/2014; Accepted On: 22/12/2014; Published On: 15/01/2014 How to cite this article: A Negi, R Singla, V Singh, Indole Based Alkaloid in Cancer: An Overview, PharmaTutor, 2014, 2(1), 76-82 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









