{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Raval Aniket*, Chauhan Sachin P., Sheth A.K, Shah Nirmal, Aundhia Chintan
Department of Pharmacy, Sumandeep Vidyapeeth University,
At & Po Pipariya, Ta. - Waghodia, Dist. Vadodara, Gujarat, India
aniket_raval2006@yahoo.co.in
ABSTRACT:
The aim of present investigation is to formulate evaluate and optimization of colon targeted pellets bearing mebendazole, benzimidazole derivative with broad spectrum of anthelminthic activity. It is highly effective against adult and larval stages of ascaris lumbricoids, hook worms and indicated for the treatment of nematode infestation. Pellets were prepared by extrusion spheronization process using microcrystalline cellulose as spheronizing aid, natural polysaccharide pectin as binders in three different percentages i.e 5%, 10% and 15% and glycerine as plasticizer. Further study was carried out to select the natural polysaccharide for formulation of colon targeted pellets i.e. Pectine, Xanthan gum and Guar gum. The formulation were prepared with optimized constant process parameters i.e. Percentage LOD 10%, Spheronization time 3 minutes and Speed of spheronization 700-1200 rpm. Prepared A1 – A9 batches were then evaluated by their micromeritic properties like tapped density, carr’s index, hausner, S ratio, angle of repose and characterized by microscopic study, % yield, hardness, friability, % assay and dissolution study was carried out and compared with marketed formulation by statistical analysis similarity factor (f2). The batch A5 is having 10% pectin, 18% MCC and 20% mebendazole shows (22.20±2.05) % carr’s index, (1.22±0.04) hausner’s ratio, (26.65±1.15) angle of repose, (88.2±2.36) % yield, (3.96±0.46) hardness, (0.23±0.03) % friability (88.47±3.26) % assay and (99.81±3.80) % drug release after 10 hours. Pellets equivalent to 300mg of mebendazole were then filled in capsules and capsules coated with 12.5% w/v Eudragit S 100 using optimized 4- 5 ml/min spray rate, 15 rpm pan speed and 40±5°C coating inlet temperature and then optimized for % weight gain in four different trials. W2 batch having 10% weight gain were then evaluated by % cumulative drug release, disintegration test in 0.1 N HCl shows (99.73±3.34) % CDR, intact after 12 hours. The pellets of batch A5 and the enteric coated capsule with 10 % weight gain were packed in aluminum pouch and charged for accelerated stability studies at (40°C±2°C) and (75%±5%) RH for 1 month in a stability chamber shows no change in the dissolution profile at (40°C±2°C) and (75%±5%) RH storage condition.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2262
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 10 How to cite this article: A Raval, SP Chauhan, AK Sheth, N Shah, C Aundhia; Formulation Evaluation and Optimization of Mebendazole Colon Targeted Sustain Release Pellets by Extrusion Spheronization; PharmaTutor; 2014; 2(10); 108-128 |
INTRODUCTION:
The oral route is considered to be most convenient for administration of drugs to patients. Oral administration of conventional dosage forms normally dissolves in the stomach fluid or intestinal fluid and absorb from these regions of the gastrointestinal tract (GIT) depends upon the physicochemical properties of the drug. It is a serious drawback in conditions where localized delivery of the drugs in the colon is required or in conditions where a drug needs to be protected from the hostile environment of upper GIT. Dosage forms that deliver drugs into the colon rather than upper GIT prefers number of advantages. A traditional oral sustained release formulation releases most of the drug at the colon, thus the drug should have absorption window either in the colon or throughout the gastrointestinal tract.[1]
Mebendazole comes under the category Anhtelminthic. Mebendazole is benzimidazole derivative with broad spectrum of anthelminthic activity. It is highly effective against adult and larval stages of ascaris lumbricoids, enterobious vermicularis, and hookworms. Recent in vitro studies have reported that mebendazole is more effective than metronidazole in killing giardia lamblia. The dose of mebendazole is 100 mg twice a day (200mg), having a bioavailability less than 20%. Its tmaxis 0.5 to 7 hrs and Cmaxis 0.03mcg/ml. Its half life is 2.5 to 5 hrs. It is short half life which favors to development of sustained release.[2] Natural polysaccharides like pectine, guar gum, xanthan gum are now extensively used for the development of solid dosage forms for delivery of drug to the colon. The rational for the development of a polysaccharide based delivery system for colon in the presence of large amounts of polysaccharides in the human colon. The colon is inhibited by a large number and variety of bacteria which secrete many enzymes e.g. ß-D glucosidase, ß-Dgalactocidase, amylase, pectinase, dextranase, etc.[3]
Report suggests that drug carrier system larger than 200 µm possess very low gastric transit time due to physiological condition of the bowel in colitis and for this reason and considering the selective uptake of micron or submicron particles by cancerous and inflamed cells/tissues. Multiparticulate approach based on pellets, granules, microsphere or nanoparticle type formulation is expected better pharmacological effect in the colon. Pellets can be formulated with size near to 1mm. Since the drughas shorter half life 2.5 to 5.0 hours,it is primarily metabolized hepatically into its inactive form and having 20% bioavailability when given orally. So, By formulating the colon targeting SR pellets of Mebendazole which contains biodegradable polysaccharides as sustain release binding agent prevent first pass metabolism, provide increase residence time resulting in prolonged drug delivery in colon and improve patient compliance by reducing dosing frequency.
[adsense:468x15:2204050025]
MATERIALS AND METHODS:
Table 1: Materials used in present investigation:
|
Mebendazole |
Welable Healthcare, Mehsana |
|
Pectin |
S.D. Fine Chemicals Ltd |
|
Xanthan gum |
S.D. Fine Chemicals Ltd |
|
Guar gum |
S.D. Fine Chemicals Ltd |
|
Eudragit S 100 |
Corel Pharmaceuticals, Ahmedabad, India |
|
Glycerine |
S.D. Fine Chemicals Ltd |
|
MCC |
S.D. Fine Chemicals Ltd |
|
TEC |
S.D. Fine Chemicals Ltd |
Microcrystalline Cellulose as Spheronization aid:
In relation to the above-mentioned requirements of the wetted mass, microcrystalline cellulose (MCC) is incorporated in most formulations processed via extrusion–spheronisation, since it provides the proper rheological properties to the wetted mass for successful extrusion and spheronisation. MCC is the golden standard as extrusion–spheronisation aid based on its good binding properties that provide cohesiveness to a wetted mass containing MCC. Furthermore, it is able to absorb and retain a large quantity of water due to its large surface area and high internal porosity, thus facilitating extrusion, improving wetted mass plasticity and enhancing spheronisation. Moreover, by controlling the movement of water through the plastic mass, it prevents phase separation during extrusion or spheronisation. Due to these properties MCC-based pellets produced via extrusion–spheronization have a good sphericity, low friability, high density and smooth surface properties. Furthermore, from a processing viewpoint, relatively wide ranges of water content and processing parameters can be employed to provide pellets with acceptable quality, indicating the robustness of the formulations.[4,5]
Mechanism of Spheronization aid:
MCC is described as a ‘molecular sponge’. The MCC particles are able to retain water in a manner similar to a sponge. During extrusion these sponges are compressed, and water that is squeezed from the internal structures acts as a lubricant. After extrusion, the volume of the spongesexpands and they appear dry and brittle, which facilitates thebreaking of the extrudates during the initial phase of spheronisation.During the spheronisation phase, the sponges are densifieddue to collisions between particles and the spheronizerplate and wall, and water facilitates spheronisation of pellets.[6,7]
Characteristics of MCC:
- Water insolubility
- Large water absorption and retention capacity
- Binding properties
- Sufficiently large surface area for interaction with water and other ingredients in the powder mixture.
EUDRAGIT S 100 offers valuable advantages for enteric coatings:[8]
- PH-dependent drug release
- Protection of actives sensitive to gastric fluid
- Protection of gastric mucosa from aggressive actives
- Increase in drug effectiveness
- Good storage stability
- GI and colon targeting
METHODOLOGY:
Preformulation study:[9]
It is the first step in rational development of dosage forms of drug substance. Preformulation testing is defined as investigation of physical and chemical properties of a drug substance alone and when combined with excipients. The overall objective of preformulation testing is to generate information useful to the formulator in developing stable and bioavailable dosage forms that can be mass-produced.
a)Organoleptic properties:[9]
The drug sample was evaluated for its colour, taste and odour.
b) Melting point determination:[10]
Melting point of the drug sample was determined by capillary method by using melting point apparatus.
c) Solubility analysis:
Solubility analysis was carried out for the selection of suitable solvent for further processing to formulation. It was carried out by making saturated solution of drug in various solvents separately, then filtered it and analyse by UV spectrophotometric technique.
d) Determination of λmax :
Determination of λmax of drug (mebendazole) is carried out by screening method. In this method making a suitable diluted solution of mebendazole in suitable solvent and scanned for the maximum absorbance in the range of 200 nm – 400 nm on Shimadzu 1700 UV/Vis double beam spectrophotometer.
e) Determination of Calibration curve:
1) Standard calibration curve of Mebendazole in 0.1N HCl + 1% SLS
Calibration curve of drug was taken in 0.1N HCl + 1% SLS. Absorbance was measured at λmax 291 nm using UV visible double beam spectrophotometer of solution.
2) Standard calibration curve of Mebendazole in phosphate buffer pH 7.4
Calibration curve of drug was taken in phosphate buffer pH 7.4. Absorbance was measured at λmax 291 nm using UV visible double beam spectrophotometer of solution.
f) Drug excipient compatibility Study:
1)Fourier-transform infrared spectroscopic study:
Infrared spectra of pure drug, polymer, as well as for combination of drug-polymer were taken by KBr pellet technique and were recorded in the range of 4000 – 400 cm-1 by using FT-IR Spectrophotometer.
2) Differential Scanning Calorimetric (DSC) study:
All the samples were tasted on DSC-60 Shimadzu by controlled heating at the rate 20oC/min in air environment in range of 50oC to 300o c.
Preparation of pellets:[11]
The wet extrusion process can be batch or continuous operation and consists of the following steps:
1. Mixing
First of all weigh accurately as per quantity of drug, MCC, DCP and mix well in motor pistal. Then add binder solution as per required and make a dump mass. Here different binders were used like pectin, xanthan gum and guar gum. These all binders are natural polysaccharides and natural polysaccharides are completely digested by colonic enzymes.
2. Extrusion
Then this mass is put in extruder for getting extrudes as shown in figure1.

3. Spheronization
The wet extrudates are placed in an oven for optimum % LOD (Loss on Drying). If there was higher moisture then extrudes stick with each other and if it was less then there were chances of fine particles. After this stage extrudes were placed in spheronizer for getting sphere pellets as shown in figure 2.

4. Drying
These wet spheres are then transferred to a FBD dryer for drying process.
Preliminary trial batches for optimization of process variables:
Fixed parameters:
· Extruder screen size: 0.87mm
· Extruder speed: 60rpm
· Final pellets drying temperature: 50oC
· Final pellets drying time: 30minutes
Variable parameters:
· %LOD (Loss on drying) of extrudes: 10%, 15%, 20%, 25%
· Spheronization time: 120sec, 180sec, 300sec, 420sec.
·Spheronization speed: 700rpm, 1000rpm, 1300rpm
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 2 : Preliminary trial batches :
|
Batches |
Pectin |
MCC |
DCP |
Glycerin |
|
P1 |
5% |
18% |
57% |
2ml |
|
P2 |
10% |
18% |
52% |
2ml |
|
P3 |
15% |
18% |
47% |
2ml |
1) Optimization of % LOD
2) Optimization of spheronization speed.
3) Optimization of spheronization time.
By using the trial and error method the effect of %LOD, spheronization time and spheronization speed on % yield in desired size range, sphere size, and sphere shape were evaluated. By using the 20% of % LOD, spheronization time 180 seconds, and spheronization speed 1000rpm results were obtained for the % yield, sphere size, and sphere shape. Sphere size and shape was depended on the spheronization speed and % LOD in the extrudes to be spheronized, whereas the % yield was significantly affected by spheronization time.
Composition of pellet formulation:
The study continues further for selection and optimization of polymer for colon targeted pellets. The selection of polymer initial short listed based on literature survey and availability to three different natural polymer and their three different percentages. The polymers used were pectine, xanthan gum and guar gum in three different percentages i.e. 5 %, 10 % and 15%w/w. These polymers are natural polysaccharides which are completely digested by colonic enzymes e.g. ß-D glucosidase, ß-D-galactocidase, amylase, pectinase, dextranase, etcand also they are cheap in cost.
In table there was fixed the quantity of MCC, Drug and glycerin. Here glycerin was used for plasticity of extrudes. The typical composition of formulation showed in table 6.5. Prepared pellets were evaluated for micromeritic properties and flow characteristics. (Bulk density, tapped density, Carr’s index, and angle of repose).
Table 3 : Composition of pellets formulations A1-A9 :
|
Batches
|
Xanthan gum (w/w) |
Pectin (w/w) |
Guar gum (w/w) |
MCC (w/w) |
DCP (w/w) |
Drug (w/w) |
Glycerine (ml) |
|
A1 |
5% |
- |
- |
18% |
57% |
20% |
2ml |
|
A2 |
- |
5% |
- |
18% |
52% |
20% |
2ml |
|
A3 |
- |
- |
5% |
18% |
47% |
20% |
2ml |
|
A4 |
10% |
- |
- |
18% |
57% |
20% |
2ml |
|
A5 |
- |
10% |
- |
18% |
52% |
20% |
2ml |
|
A6 |
- |
- |
10% |
18% |
47% |
20% |
2ml |
|
A7 |
15% |
- |
- |
18% |
57% |
20% |
2ml |
|
A8 |
- |
15% |
- |
18% |
52% |
20% |
2ml |
|
A9 |
- |
- |
15% |
18% |
47% |
20% |
2ml |
Evaluation of pellets:
1) Tapped density = m/vt
m = Mass of pellets
vt = Tapped volume
2) Carr’s index(%) = [ρt -ρb/ρt] ×100
ρb = Bulk density
ρt = Tapped density
3) Hausner;s ratio = ρt/ρb
ρt = Tapped density
ρb = Bulk density
4) Angle of repose:
The angle of repose for the pellets of each formulation was determined by the funnel method. The angle of repose was calculated by substituting the values of the base radius ‘R’ and pile height ‘H’ in the following equation: tan θ = H / R Therefore; θ = tan–1 (H / R).[12,13]
5) SEM analysis:
Samples of pellets of A5 batches were dusted on onto silica gel applied sample holder. The samples were imaged using 15 kV electron beam.
Characterization of batches for process variables:
1)Microscopy study of pellets:
Photomicrographs of the pellets obtained from various batches were taken using a fluorescence microscope (Leica inverted fluorescence microscope).
2) Percentage yield:
It was calculated by following formula.[14]

3) Hardness of pellets:
Hardness of pellets was measured by using digital pharmatest hardness tester.[14]
4) Friability test:
Friability test for pellets was carried out using Roche friabilater
5) Assay:
Assay of the drug was performed by UV spectroscopy method. The absorption of the solution has measured at 291 nm by UV spectroscopy method.[15]
Dissolution profile of batch A1-A9 in phosphate buffer pH-7.4:
The drug release study was carried out using auto sampler dissolution test apparatus USP type 1 basket (TDT-08L, Electrolab, Mumbai, India.)at 37°C ± 0.5°C and at 100 rpm using 900 ml of phosphate buffer of pH 7.4 as dissolution medium as per USP XXVI. Percentage drug dissolved at different time intervals was calculated using the Lambert-Beer’s equation from the calibration curve.[16]
Comparison of dissolution profile by statistical analysis similarity factor (f2):
The dissolution profiles of products were compared using f2. The similarity factor is calculated by following formula, [10]
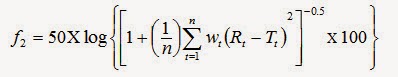
Where, n is the number of dissolution time points
Rt -The reference profile at the time point t
Tt - The test profile at the same point.
Eudragit coating on capsules of optimized batch:
Eudragit S 100 has 12.5% availability in organic solution and dissolve pH >7.
Table 4 : Optimised batch for coating process :
|
Materials |
Quantity |
|
Drug |
20% |
|
MCC |
18% |
|
DCP |
57% |
|
Pectin |
10% |
|
Guar gum |
2ml |
Table 5 : Composition of coating solution :
|
Ingredients |
Quantity |
|
Eudragit S 100 |
12.5 % |
|
Talc |
2 % |
|
Tri ethyl citrate (TEC) |
2% |
|
Methanol : Acetone (5:5) |
q.s. |
Coating process:
The hard gelatin capsules of size 0 were filled with pellets of mebendazole of the optimized batch A5. Pellets equivalent to 300mg of mebendazole were filled in each capsule.The enteric coated capsules were prepared by using conventional coating pan. Coating solution was prepared by dissolving coating polymer in to the Methanol and Acetone mixture (1:1) and uniform dispersion of coating solution was spray on the capsule bed under the following condition until desired coating thickness was obtained.
About 10 capsules of mebendazole sustained release matrix pellets were taken and allow to coating in pan coater at 15 rpm and 40oC temperature. Coating was carried out with spraying method and dried with same.[16]
Table 6: Process parameters for coating :
|
Spray rate |
4-5 ml/min |
|
Pan speed |
15 rpm |
|
Hot air inlet temperature |
40 ± 5°C |
The coated capsules were dried for 10-15 min. in coating pan. The amount of coating was done up to 5 to 20% per capsule. The % weight gain was then optimized considering the in vitro dissolution test.
Optimization of process parameters:
In process optimization study, optimization of % weight gain of capsules, inlet air temperature and pan speed were carried out.
1) Effect of different weight gain:
To know the effect of different weight gain; four trials were taken with four different % weight gains and % weight gains of the batches were 5 %, 10% , 15% and 20%.
2) Effect of different Inlet Temperature.[17]
To know the effect of different inlet air temperature three batches were taken with three different temperatures 30º, 40º and 50° C and coating process continue till 10 % weight gain achieved in each batch.[15]
3) Effect of different speed of rotating pan:
Hence coating was performed at different rotating speed of pan 5, 10, and 15 rpm at constant inlet air temperature (40° C) and coating process continued till 10 % weight gain was achieved in each batch.
Evaluation of enteric coated capsules:
After and during enteric coating procedure capsules were evaluated for % weight gain, disintegration test.
1) Weight gain:
It was calculated using following equation.
% Wg a = [(Wt a – Wt b) Wt b] ×100
Where, Wt b and Wt are the total batch weights before and after coating, respectively.
2)Disintegration test:
Disintegration testing of coated dosage forms was carried out in the six tablet basket rack USP disintegration apparatus maintained at 37°C ±2°C using dissolution medium 0.1 N HCl for first 2 hours, phosphate buffer pH 6.4 after 2 hours and phosphate buffer pH 7.4 after 3 hours.
Evaluation of optimized enteric coated batch:
It was carried out using pan coater at speed 15 RPM, inlet air temperatures kept at 40°C. Eudragit dispersion was applied until 10 % weight gain achieved.
Accelerated stability testing of the optimized Batch:
The pellets of batch A5 and the enteric coated capsule with 10 % weight gain were packed in aluminum pouch and charged for accelerated stability studies at 40°C and 75% RH for 1 month in a stability chamber.
RESULT AND DISCUSSION:
Preformulation study:
a) Organoleptic properties:
|
Table 7 - Results of organoleptic properties |
|
|
Properties |
Results |
|
Color |
Off white to slightly yellow |
|
Odor |
Amorphus |
|
Taste |
Unpleasant |
b) Determination of melting point:
Table 8 : Result of melting point :
|
Reported Melting Point |
Observed Melting Point |
|
288°C |
286°C - 290°C |
c) Solubility study :
Table 9 : Result of solubility study :
|
Solvents |
Terms |
|
|
Water |
Less than 0.05 % |
|
|
0.1N HCl |
Less than 0.05 % |
|
|
Formic Acid |
Soluble |
|
|
Phosphate buffer |
pH 6.8 |
Soluble |
|
pH 7.4 |
Soluble |
|
|
Chloroform |
Insoluble |
|
|
DCM(Dichloromethane) |
Soluble |
|
d) Determination of caliberation curve:
Table 10.1 : Calibration curve of mebendazole in 0.1 N HCL+ 1% SLS at 291 nm
|
Sr. No |
Concentration ( μg/ml) |
Absorbance |
Average absorbance |
||
|
1 |
5 |
0.170 |
0.165 |
0.175 |
0.170±0.005 |
|
2 |
10 |
||||









