{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
V.T. Iswariya*, A.Hariomprakash Rao, K. Sri Jahnavi, A.Sravanthi, P. Deepa, K. Samatha
M.R.R. College of Pharmacy,
Nadergul, Andhra Pradesh, India
*iswariyapharma@gmail.com
ABSTRACT
The technique of Solid dispersion and sublimation techniques are a promising method towards enhancing the dissolution of poorly soluble drugs. The main objective of Resperidone mouth dissolving tablets is to enhance the solubility. Several formulations of solid dispersion and sublimating tablets were prepared by using different ratio of drug sublimating agent (Camphor) and carriers (PEG 4000, Polaxomer, Mannitol). The prepared Solid dispersion and sublimating tablets were evaluated for their flow properties such as bulk density, tapped density, angle of repose, Carr’s index and Hausner’s ratio. The interaction between drug and excipients were studied by FTIR. In vitro dissolution profiles of the solid dispersion and sublimation formulations were studied and compared between sublimation and solid dispersion formulation. Among all formulations SD2 formulation was shown maximum drug release in less time.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2435
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 9 Received On: 07/04/2016; Accepted On: 02/05/2016; Published On: 01/09/2016 How to cite this article: Iswariya VT, Rao AH, Jahnavi KS, Sravanthi A, Deepa P, Samatha K; Formulation and evaluation of Oro Disintigrating tablets of Resperidone by using Sublimation and Solid Dispersion Technique; PharmaTutor; 2016; 4(9); 46-54 |
INTRODUCTION
The oral route of administration is considered as the most widely accepted route because of its convenience of self administration, compactness and easy manufacturing. But the most evident drawback of the commonly used oral dosage forms like tablets and capsules is difficulty in swallowing, leading to patients incompliance particularly in case of paediatric and geriatric patients, but it also applies to people who are ill in bed and to those active working patients who are busy or travelling, especially those who have no access to water.
For these reasons, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Oral dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people. [1]
An orally disintegrating tablet (ODT)is a solid dosage form that contains medicinal substances and disintegrates rapidly (within seconds) without water when placed on the tongue. The drug is released, dissolved, or dispersed in the saliva, and then swallowed and absorbed across the GIT. [2]
US FDA defined MDT tablets as “A solid dosage form containing medicinal substances which disintegrates rapidly usually within a matter of seconds, when placed upon the tongue”[3].
Recently European Pharmacopoeia used the term ‘Orodispersible tablet’ [4] as a tablet that is to be placed in the mouth where it disperses rapidly before swallowing.
Orally disintegrating tablets are also called as mouth-dissolving tablets, fast disintegrating tablets, fast dissolving tablets, orodispersible tablets, rapimelts, porous tablets, quick dissolving tablet.
The US Food and Drug Administration responded to this challenge with the 2008 publication of Guidance for Industry: Orally Disintegrating Tablets (Rosie et al., 2009)[5].Three main points stand out in the final guidance:
- MDTs should have an in vitro disintegration time of approximately 30 sec or less.
- Generally, the MDT tablet weight should not exceed 500 mg, although the combined influence of tablet weight, size, and component solubility all factor into the acceptability of an MDT for both patients and regulators.
- The guidance serves to define the upper limits of the MDT category, but it does not supersede or replace the original regulatory definition mentioned. In other words, disintegration within a matter of seconds remains the target for an MDT.
NEED TO DEVELOP MDT[6]:
The need for one of the non-invasive delivery system i.e., orally disintegrating tablets persists due to patients’ poor acceptance of, and compliance with, existing delivery regimes, limited market size for drug companies and drug uses, coupled with high cost of disease management.
DESIRED CHARACTERISTICS AND CHALLENGES TO DEVELOP ORODISPERSIBLE TABLETS[7-9].
RAPID DISINTEGRATION: Rapid disintegration is a desired characteristic of MDT where it rapidly disintegrates in mouth in matter of seconds. Selection of super disintegrants which tends the drug to release fast is a challenging part of formulation scientist.
PALATABILITY : As most of the drugs are bitter in taste or tasteless, effective taste masking technology should be adopted to develop orodispersible tablet as they disintegrate or dissolve in patient’s oral cavity. Hence taste masking of orodispersible tablets becomes challenging as well as a desired characteristic for better mouth feel and patient’s compliance.
MECHANICAL STRENGTH: The orodispersible tablet should be formulated in such a way that it disintegrates rapidly in fraction of seconds in oral cavity which is a desired characteristic for patients who have swallowing problem and should hold the mechanical integrity of tablet to avoid friability and special packing which is challenging to formulators.
HYGROSCOPICITY: Several orally disintegrating dosage forms are hygroscopic and cannot maintain physical integrity under normal conditions of temperature and humidity. Hence, the formulators should develop a system to maintain low sensitivity to environment conditions which is a challenging as well as desired character to attain.
AMOUNT OF DRUG: The amount of drug incorporated as an orodispersible tablet should give sufficient response as well as it must be lower than 400mg for insoluble drugs and less than 60 mg for soluble drugs.
SIZE OF THE TABLET: The degree of ease when taking a tablet depends on its size. The size of the tablet should be such that it should be easy to swallow and to handle and for good packing.
CRITERIA FOR SELECTION OF DRUG TO DEVELOP MDT [10-12]:
An MDT may have varying degrees of pregastric absorption of drugs and thus, the pharmacokinetic profiles of drugs will vary, therefore, the MDTs will not be bioequivalent to the conventional dosage forms.
The ideal characteristics of a drug to develop as an MDT include:
- No bitter taste.
- Small to moderate molecular weight.
- Good stability in water and saliva.
- Partially non-ionized at the oral cavities pH.
- Ability to diffuse and partition into the epithelium of the upper GIT (log P > 1, or preferably > 2).
- Ability to permeate oral mucosal tissue.
- Dose should be low as possible.
Unsuitable drug characteristic for MDTs:
- Short half-life and frequent dosing.
- Very bitter or otherwise unacceptable taste because taste masking cannot be achieved.
- Required controlled or sustained release.
SELECTION OF SUPER DISINTEGRANTS:
Super disintegrants not only affect the rate of disintegration, but when used at higher concentrations they also affect mouth feel, tablet hardness and friability (Rakesh et al., 2010)[13]. Superdisintegrants are selected according to critical concentration of disintegrant. Below this concentration, the tablet disintegration time is inversely proportional to the concentration of the superdisintegrant .Hence, various ideal characteristics of super disintegrants should be considered while selecting for a particular formulation.
- Produce rapid disintegration.
- Be compactable enough to produce less-friable tablets.
- Produce good mouth feel to the patient. Thus, small particle size is preferred to achieve patient’s compliance
- Should have good flow properties to improve the flow ability of the total blend.
MECHANISM OF SUPER DISINTEGRANTS:
There are major mechanisms for tablets disintegration as follows (Khuldeep et al., 2010)14.
Swelling: Perhaps the most widely accepted general mechanism of action for tablet disintegration is swelling. Tablets with high porosity show poor disintegration due to lack of adequate swelling force. On the other hand, sufficient swelling force is exerted in the tablet with low porosity. It is worthwhile to note that if the packing fraction is very high, fluid is unable to penetrate in the tablet and disintegration is again slows down.
POROSITY AND CAPILLARY ACTION (WICKING): Disintegration by capillary action is always the first step. When we put the tablet into suitable aqueous medium, the medium penetrates into the tablet and replaces the air adsorbed on the particles, which weakens the intermolecular bond and breaks the tablet into fine particles. Water uptake by tablet depends upon hydrophilicity of the drug /excipients and on tableting conditions.
For these types of disintegrants maintenance of porous structure and low interfacial tension towards aqueous fluid is necessary which helps in disintegration by creating a hydrophilic network around the drug particles.
DUE TODISINTEGRATING PARTICLE/PARTICLE REPULSIVE FORCES: Another mechanism of disintegration attempts to explain the swelling of tablet made with ‘non swellable’ disintegrants. Guyot-Hermann has proposed a particle repulsion theory based on the observation that non swelling particle also cause disintegration of tablets. The electric repulsive forces between particles are the mechanism of disintegration and water is required for it. Researchers found that repulsion is secondary to wicking.
DUE TO DEFORMATION: During tablet compression, disintegrated particles get deformed and these deformed particles get into their normal structure when they come in contact with aqueous media or water. Occasionally, the swelling capacity of starch was improved when granules were extensively deformed during compression. This increase in size of the deformed particles produces a breakup of the tablet. This may be a mechanism of starch and has only recently begun to be studied.
BECAUSE OF HEAT OF WETTING (AIR EXPANSION): When disintegrants with exothermic properties gets wetted, localized stress is generated due to capillary air expansion, which helps in disintegration of tablet. This explanation, however, is limited to only a few types of disintegrants and cannot describe the action of most modern disintegrating agents.
DUE TO RELEASE OF GASES: Carbon dioxide released within tablets on wetting due to interaction between bicarbonate and carbonate with citric acid or tartaric acid. The tablet disintegrates due to generation of pressure within the tablet. This effervescent mixture is used when pharmacist needs to formulate very rapidly dissolving tablets or fast disintegrating tablet. As these disintegrants are highly sensitive to small changes in humidity level and temperature, strict control of environment is required during manufacturing of the tablets. The effervescent blend is either added immediately prior to compression or can be added in to two separate fraction of formulation.
BY ENZYMATIC REACTION: Here, enzymes present in the body act as disintegrants. These enzymes destroy the binding action of binder and helps in disintegration. Actually due to swelling, pressure exerted in the outer direction or radial direction, it causes tablet to burst or the accelerated absorption of water leading to an enormous increase in the volume of granules to promote disintegration.

Figure: 1. Disintegration of tablet by wicking and swelling

Figure: 2. Disintegration of tablet by deformation and repulsion.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
EXAMPLES OF SUPERDISINTEGRANTS:
Table.1 Examples of superdisintegrants.
|
Brand Name
|
Common Name |
Classification
|
Functional Category
|
Properties
|
EMC at 25ºC/ 90%RH |
Typical Uses
|
|
CL-Kollidon
|
Crospovidone |
Polyvinyl Pyrrolidone |
Tablet super Disintegrant |
Swelling (18% in10s), (45% in 20s) |
62% |
Disintegrant (Dry and Wet granulation) |
|
Ac-DiSol |
Croscarmellose sodium |
Cellulose, carboxymethyl ether, sodium saltcrosslinked |
Tablet and capsule disintegrant
|
Wicking and swelling (12% in 10s), (23% in 20s) |
88% |
Disintegrant for capsules, tablets and granules. |
|
ExplotabPrimojel |
Sodium starch Glycolate |
Sodium carboxymethyl Starch |
Tablet and capsule super disintegrant |
Swelling capacity (300 times) |
- |
Disintegrant (Dry and Wet granulation) |
|
L-HPC |
Hydroxypropyl cellulose(low substituted) |
Cellulose, 2-hydroxypropyl Ether |
Tablet and capsule super disintegrant |
Swelling (13% in 10s), (50% in 20s) |
37% |
Disintegrant and Binder in wet Granulation |
|
Amberlite IRP 88 |
Polacrillin Potassium |
Cation exchange resin |
Diluent and Disintegrant |
Swelling |
- |
Disintegrant |
Materials
Resperidone, Poloxomer, PEG 4000, Mannitol, Explotab, Mg.stearate, Microcrystalline Cellulose, Talc, Polyplasdone XL, Camphor
Formulation development for solid dispersion:
Solid dispersions were prepared by solvent evaporation method. Methanol was used as solvent. Resperidone and Water soluble polymers such as Polaxomer, Mannitol and PEG 4000 were selected as carriers. Drug and polymers were taken in 1:1 ratio stated in the formulation chart (Table 2). The prepared solid dispersions were passed through the sieve no 20 to get uniform sized particles. The solid dipersions were mixed with required quantities of super disintegrants, diluent, lubricant and glidant (shown in Table 3). The blend was evaluated for precompression parameters.
Table 2: Preparation of solid dispersion showing various compositions
|
|
SD1 |
SD2 |
SD3 |
|
Drug |
20 |
20 |
20 |
|
Polaxomer |
20 |
-- |
-- |
|
PEG 4000 |
-- |
20 |
-- |
|
Mannitol |
-- |
-- |
20 |
Table 3: Formulation of fast dissolving tablet by using solid dispersion
|
|
SD1 |
SD2 |
SD3 |
|
Equivalent to 2mg |
4 |
4 |
4 |
|
Explotab |
7.5 |
7.5 |
7.5 |
|
Mg.stearate |
2 |
2 |
2 |
|
Talc |
2 |
2 |
2 |
|
MCC |
QS |
QS |
QS |
|
Total wt |
100 |
100 |
100 |
Formulation Development for Sublimation method:
Optimization of camphor concentration:Concentration of camphor should be optimized initially by keeping all the ingredients constant. Camphor was used in the concentration of 2.5, 5 and 10 mg. after preparation of tablets kept in hot air oven at 40-60 0c then observed pores forming on tablets. Whenever increase the concentration of sublimating agent increase the pores on tablets. Among 3 formulations C3 formulation was showed more pores on tablets.
|
Ingredients |
C1 |
C2 |
C3 |
|
Resperidone |
2 |
2 |
2 |
|
Polyplasdone XL |
5 |
5 |
5 |
|
Camphor |
2.5 |
5 |
10 |
|
Mg.stearate |
2 |
2 |
2 |
|
Talc |
2 |
2 |
2 |
|
MCC |
Q.S |
Q.S |
Q.S |
|
Total weight |
100 |
100 |
100 |
* all quantities were taken in mg
Table: 4 Optimisation of camphor concentration
Preparation of Resperidone Mouth dissolving tablets by sublimation method
- Drug different concentrations of super disintegrate and optimized concentration of camphor were accurately weighed and passed through a 20-mesh screen to get uniform size particles and mixed in a glass mortar for 15 minutes.
- The obtained blend was lubricated with magnisium stearate and Talc was added and mixing was continued for further 5 minutes.
The resultant mixture was directly compressed into tablets by using 6mm round flat faced punch of rotary tablet compression machine.
Table: 5 Formulation of Sublimation conventional tablets
|
Ingredients |
F1 |
F2 |
F3 |
F4 |
|
Resperidone |
2 |
2 |
2 |
2 |
|
Polyplasdone XL |
2.5 |
5 |
-- |
-- |
|
Explotab |
-- |
-- |
2.5 |
5 |
|
Camphor |
10 |
10 |
10 |
10 |
|
Mg.stearate |
2 |
2 |
2 |
2 |
|
Talc |
2 |
2 |
2 |
2 |
|
MCC |
QS |
QS |
QS |
QS |
|
Total weight |
100 |
100 |
100 |
100 |
* all quantities were taken in mg
RESULTS AND DISCUSSION
Analytical Method Development
Construction of calibration curve forResperidone:
The λmax of phosphate buffer pH 6.8 of Resperidone were found to be at 234 nm. Standard graphs of Resperidone in phosphate buffer pH 6.8 were shown in Table 6. Good linearity was observed with concentration verses absorbance. Its R2 value in 0.1N HCl and phosphate buffer pH 6.8 was 0.999 which were very nearer to ‘1’ and so obeys “Beer -Lambert's law".
Table: 6. calibration curve of Resperidone inphosphate buffer pH 6.8
|
Concentration(µg/ml) |
Absorbance |
|
0 |
0 |
|
5 |
0.145 |
|
10 |
0.309 |
|
15 |
0.439 |
|
20 |
0.585 |
|
25 |
0.747 |
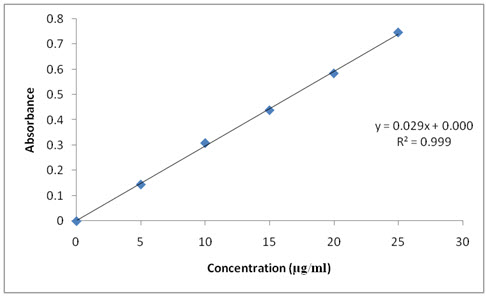
Figure: 3 Calibration curve of Resperidone in phosphate bufferpH 6.8
Drug Excipient Interactions
Fourier transform infrared (FTIR) spectroscopy studies:
The pure drug the optimized Solid dispersion formulations were subjected to FTIR studies. The results were showed that there is no interaction between the drug and excipients.
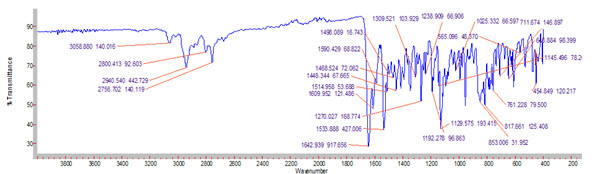
Figure 4: FTIR graph of Pure drug (Resperidone)
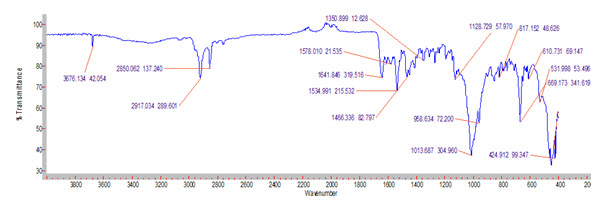
Figure 5: FTIR graph of ResperidoneOptimised formulation
There is no appearence or disappearence of ftir peaks. Its evident that there was no interaction between the drug and excipients. its perfectly compatible of drug and excipients.
Micromeric properties:
The micrometric properties of blend of Resperidonesoilddispersion were characterized with respect to angle of repose, bulk density, tapped density, Carr’s index and Hausner’s ratio. Angle of repose was less than 280, Carr’s index values were 10 to 17 for the precompression blend of all the batches indicating good to fair flowability and compressibility. Hausner’s ratio was less than 1.2 for all the batches indicating good flow properties.
Table: 7. Evaluation of pre compression parameters of solid dispersion tablets.
|
Formulation Code |
Angle of repose(θ) |
Bulk density (gm/cc) |
Tapped density (gm/cc) |
Carr's index |
Hausner ratio |
|
SD1 |
29.74 |
0.29 |
0.33 |
12.12 |
1.13 |
|
SD2 |
26.03 |
0.32 |
0.40 |
15.5 |
1.05 |
|
SD3 |
35.73 |
0.35 |
0.45 |
22.2 |
1.21 |
Table: 8 Evaluation of pre compression parameters of sublimation tablets.
|
Formulation Code |
Angle of repose(θ) |
Bulk density (gm/cc) |
Tapped density (gm/cc) |
Carr's index |
Hausner ratio |
|
F1 |
28.74 |
0.32 |
0.41 |
12.12 |
1.25 |
|
F2 |
31.03 |
0.33 |
0.41 |
15.5 |
1.24 |
|
F3 |
32.73 |
0.35 |
0.40 |
22.2 |
1.14 |
|
F4 |
26.63 |
0.35 |
0.41 |
13.19 |
1.17 |
Post compression parameters:
The results of the weight variation, hardness, thickness, friability, and drug content of the solid dispersion and sublimation tablets were given in Table 9,10. All the tablets of different batches complied with the official requirement of weight variation as their weight variation passes the limits. The hardness of the tablets ranged from 2 to 3 kg/cm2 and the friability values were less than 1% indicating that the tablets were compact and hard. The thickness of the tablets ranged between 2 to 3 mm. All the formulations satisfied the content of the drug as they contained 96-100% of Respiridoneand good uniformity in drug content was observed. Thus all the physical attributes of the prepared tablets were found to be practically within control limits.
Table: 9 Evaluation of post compression parameters of solid dispersion tablets
|
Formulation code |
Average Weight (mg) |
Thickness (mm) |
Hardness (kg/cm2 ) |
Friability |
Disintegration time (sec) |
Content uniformity (%) |
|
SD1 |
98 |
2.2 |
2.5 |
0.39 |
58 |
96.31 |
|
SD2 |
99 |
2.1 |
2.1 |
0.29 |
34 |
98.34 |
|
SD3 |
101 |
2.4 |
2.7 |
0.32 |
47 |
97.36 |
Table : 10 Evaluation of post compression parameters of sublimation tablets
|
Formulation code |
Average Weight (mg) |
Thickness (mm) |
Hardness (kg/cm2 ) |
Friability |
Disintegration time (sec) |
Content uniformity (%) |
|
F1 |
96 |
2.3 |
2.5 |
0.39 |
48 |
94.31 |
|
F2 |
99 |
2.4 |
2.1 |
0.29 |
32 |
98.34 |
|
F3 |
101 |
2.2 |
2.7 |
0.32 |
51 |
95.36 |
|
F4 |
99 |
2.3 |
2.3 |
0.39 |
46 |
98.63 |
Table: 11 Characterization of formulated Solid dispersion tablets.
|
Formulation |
Wetting time (sec) |
Water absorption ratio(%) |
Dispersion time(sec) |
|
SD1 |
12 |
91.2 |
20 |
|
SD2 |
10 |
96.5 |
18 |
|
SD3 |
15 |
89.2 |
23 |
Table: 12 Characterization of formulated sublimation tablets.
|
Formulation |
Wetting time (sec) |
Water absorption ratio(%) |
Dispersion time(sec) |
|
F1 |
15 |
91.2 |
25 |
|
F2 |
13 |
95.4 |
21 |
|
F3 |
16 |
88.2 |
26 |
|
F4 |
17 |
93.2 |
24 |
From the above pre and post compression of solid dispersion and sublimation tablets of tables all the required evaluation tests were found to be within limit.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
In vitro Dissolution Studies
All the solid dispersion and sublimation formulations of Resperidonewere subjected to in vitro dissolution studies, these studies were carried out using phosphate buffer pH 6.8 by using dissolution apparatus type II.
The dissolution profile of Resperidone tablets were compared between solid dispersion and sublimation tablets. The Resperidonesolid dispersible tablets showed better release inphosphate buffer pH 6.8 than in sublimation, in that SD2 showed good drug release i.e., 99.89 at 20 minutes compared to sublimation tablet 97.2 at 30 min.(shown in table13 and 14)
Table: 13 In vitro dissolution studies of formulatedsolid dispersion tablets
|
Time(min) |
SD1 |
SD2 |
SD3 |
|
0 |
0 |
0 |
0 |
|
5 |
29.86 |
36.33 |
21.5 |
|
10 |
42.72 |
62.18 |
56.8 |
|
15 |
68.75 |
99.89 |
58.75 |
|
20 |
80.35 |
99.89 |
70.35 |
|
30 |
87.94 |
|
77.94 |
|
45 |
96.24 |
|
89.5 |
|
60 |
96.24 |
|
91.3 |
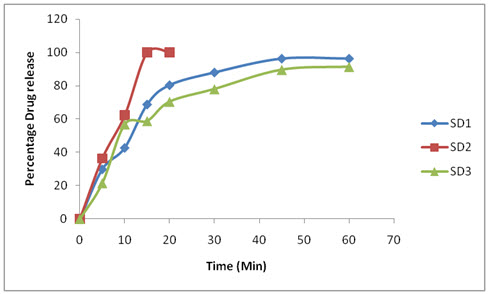
Figure 6: In-vitro release of Resperidone SD1,SD2,SD3.
Table: 14In-vitro dissolution studies of formulatedsublimation tablets
|
Time(min) |
F1 |
F2 |
F3 |
F4 |
|
0 |
0 |
0 |
0 |
0 |
|
5 |
32.86 |
44.33 |
21.5 |
30.47 |
|
10 |
54.56 |
59.89 |
32.8 |
38.48 |
|
15 |
69.75 |
88.2 |
49.75 |
52.68 |
|
20 |
73.34 |
97.2 |
52.32 |
69.46 |
|
30 |
81.94 |
97.2 |
58.94 |
82.17 |
|
45 |
96.5 |
|
63.28 |
96.58 |
|
60 |
96.5 |
|
88.14 |
96.58 |
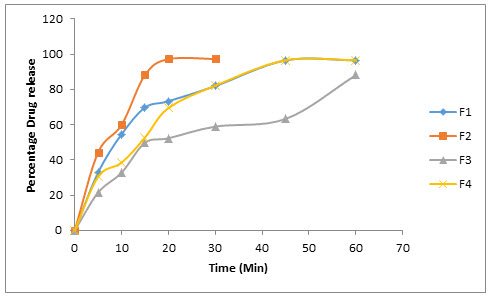
Figure 7: In - vitro release of Resperidone F1,F2,F3,F4.
So the optimised formulation is SD2. Because showed good drug release i.e., 99.89 at 20 minutes compared to sublimation tablet 97.2 at 30 min.
CONCLUSION
Risperidone, a benzisoxazole derivative, is an atypical antipsychotic drug with high affinity for 5-hydrotryptamine (5-HT) and dopamine D2 receptors. It is used primarily in the management of schizophrenia, inappropriate behavior in severe dementia and manic episodes associated with bipolar I disorder. Risperidone is effective for treating the positive and negative symptoms of schizophrenia owing to its affinity for its “loose” binding affinity for dopamine D2 receptors and additional 5-HT antagonism compared to first generation antipsychotics, which are strong, non-specific dopamine D2 receptor antagonists.
The formulated Resperidone tablets were performed effective way for enhancement of water solubility by using solid dispersion and sublimation technique. The comparative study of Resperidone tablets has given increase solubility with solid dispersion when compared with sublimation method. This novel approach to the formulation may be helpful to improve oralbioavailability. Further the efficacy of the developed formulations has to be assessed by pharmacokinetic studies in humans.
Resperidonesoild dispersion, Resperidone Sublimation conventional tablets were characterized with respect to angle of repose, bulk density, tapped density, Carr’s index and Hausner’s ratio. Passes the all pre - compression parameters and post compression parameters of solid dispersion formulations, Sublimation formulations. Wetting time is less for SD2 solid dispersion formulation is 10 sec. F2 sublimation formulation is 13 sec. Water absorption ratio (%) is more for formulation SD2 is 96.5% while F2 formulation having 95.4% water absorption ratio.
Release of Resperidone in SD2 formulation of solid dispersion was about 99% which shows maximum drug release when compared with F2 formulation of sublimation.
REFERENCES
1. Jaysweh J Harani, Dhaval A Rathod, Kantilal R Vadila, Orally disintegrating tablets,A review,Tropial Journal of Pharm.Sciences, 2009, 8(2), 161-172.
2. V.B.Yadav, A.V.Yadav, Liquisolid granulation technique for tablet manufacturing, an overview, Journal of Pharmacy Research,2009, 2(4), 670-674.
3. Spireas S, Bolton M,Liquisolid systems and methods of preparing same, U.S. Patent 5,968,550, 1999.
4. Ellsworth AJ, Witt DM, Dugdale DC, Medical Drug Reference,Elsevier science, Missouri, 2003, 610‐612.
5. Rosie et al., 2009Orally Disintegrating Tablets The Effect of Recent FDA Guidance on ODT Technologies and Applications
6. Subrahmanyam, C. V. S. Dissolution. In Textbook of Physical Pharmaceutics, 2nd ed.; Jain, M. K., Ed.; VallabhPrakashan: Delhi, India, 2000; pp 1, 92.
7. Ahmad Zaheer et al., Solubility enhancement of poorly water soluble drugs, a review IJPT, 2011, 3(1), 807-82.
8. Brahamankar D. M; Jaiswal S. B; Bioavailability and Bioequivalence,In Biopharmaceutics and Pharmacokinetics, A Treatise, 1st edition, Vallabh Prakashan: Delhi, India, 1995, 298-299.
9. Sekiguchi K, Obi N, Studies on absorption of eutectic mixtures, I. A comparison of the behavior of eutectic mixtures of sulphathiazole and that of ordinary sulphathiazole in man,Chem. Pharm. Bull,1961, 9, 866–872.
10. Fahmy RH, Kassem MA, Enhancement of famotidine dissolution rate through liquisolid tablet formulation: In vitro and Invivo evaluation,Eur. J. Pharm. Biopharm,2008, 69,993-1003.
11. Spiras S,Liquisolid systems and methods for preparing same,United States patent, 6,423,339 B1, (2002).
12. Ajit S. Kulkarni, Nagesh H. et al.,Liquisolid Systems: A Review .International Journal of Pharmaceutical Sciences and Nanotechnology, 2010, 3(1), 795-802.
13. Rakesh Pahwa et al., Orally Disintegrating Tablets - Friendly to Pediatrics and Geriatrics, “Scholar Research Library, Archives of Applied Science Research”, 2010, 2 (2): 35-48
14. Kuldeep M et al. An Emerging Trend In Oral Drug Delivery Technology: Rapid Disintegrating Tablets. Journal of Pharmaceutical Science and Technology. 2010; 2(10): 318-329.
15. Spireas SS, Jarowski CI, Rohera BD. Powder Solution technology, Principles and Mechanism,Pharma Research, 1992, 9, 1351 – 1358.
16. Liao CC, Physicochemical properties of selected powdered drug solutions, Ph.D. thesis, St.John’s university, Jamaica, NY, 1983.
17. Martin AN, Swarbrick J, Cammarata A, Physical Pharmacy, Lea &Febiger, Philadelphia,1983; 445-468.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









