{ DOWNLOAD AS PDF }
 ABOUT AUTHOR
ABOUT AUTHOR
S. Z. Chemate, Vaishali N. Garje*, Shubhada A. Gayake
Department of Pharmaceutics,
Padmashri Dr. Vithhalrao Vikhe Patil Foundation’s College of Pharmacy,
Ahmednagar, Maharashtra, India
*garjev@gmail.com
ABSTRACT
The aim of present investigation was to design and evaluate, mucoadhesive buccal patch of Metformin hydrochloride, a BCS class II drug, to provide unidirectional sustained drug delivery to the buccal mucosa that has potential to enhance the bioavailability. The patches were prepared using HPMC K4M as a polymer, polyethylene glycol 400 as a plasticizer, by solvent casting technique. The patches, which were prepared by the solvent casting method, were smooth and elegant in appearance; were uniform in thickness, weight and drug content; showed no visible cracks; and showed good folding endurance. The amount of polymer, which significantly influenced characteristics like swelling index, mucoadhesive strength, diffusion study
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2418
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 7 Received On: 08/02/2016; Accepted On: 22/02/2016; Published On: 01/07/2016 How to cite this article: Chemate SZ, Garje VN, Gayake SA; Formulation and Evaluation of Metformin Hydrochloride buccal patch; PharmaTutor; 2016; 4(7); 52-58 |
INTRODUCTION
In recent years, delivery of therapeutic agents through various transmucosal routes has received significant attention owing to the agents presystemic metabolism or instability in the acidic environment associated with oral administration.[1,2] The oral drug delivery is considered to be the most preferred route by majority of the patients amongst the various available roués of drug delivery. Oral transmucosal drug delivery can be achieved through 1 of the 3 types of oral mucosa: sublingual, gingival, and buccal. Absorption of therapeutic agents from the oral cavity provides a direct entry for such agents into the systemic circulation, thereby avoiding first-pass hepatic metabolism and gastrointestinal degradation.[3,4] However, the buccal route of drug delivery has received the most attention because of its unique advantages over the other oral transmucosal routes. [5] An ideal patch should be flexible, elastic, and soft yet strong enough to withstand breakages due to stress from activities in the mouth. Moreover, it must also possess good mucoadhesive strength so that it is retained in the mouth for the desired duration. To prevent discomfort, swelling of the patch should not be too extensive. Recently developed mucoadhesive buccal delivery systems such as adhesive tablet, films, patches, disks, strips, ointment, gel, and creams. Tablets, films and patches appear to be the most preferred formulations.
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. The absolute bioavailability of a Metformin HCL under fasting conditions is approximately 50 to 60%. Gastrointestinal absorption occurs mainly in the upper intestine and is complete at 6 hours, with peak plasma concentrations (Cmax) reached after 2 to 3 hours.[5-8]
MATERIAL AND METHOD
Material: The drug Metformin hydrochloride was procured from Balaji Drugs. Whereas HPMC K4M was procured from OZONE Internationals, Mumbai. Polyethylene glycol 400 was obtained from Merck Specialities.
Method:
Preparation of Mucoadhesive Buccal Patches
The buccal patches were prepared by solvent casting technique. The polymers were dissolved in casting solvent (10 ml) and plasticizer (30%) were incorporated then calculated amount of drug dissolved in methanol (2.5 ml) was added in polymeric solution with continuous stirring till homogeneous mixture was formed. This 12.5 ml of solution was poured with in glass mould which was placed on a mercury substrate in a petridish and allowed to drying for 5-6 hr. at 600c in oven till a flexible film was formed. The composition of buccal patches is shown in table 1.
TABLE NO.1 Formula for preparation of buccal patches
|
SR.NO. |
Ingredient |
F1 |
F2 |
F3 |
|
1 |
Metformin Hydrochloride |
40 mg |
40 mg |
40 mg |
|
2 |
HPMC K4M |
2.4%*¹ |
4.4%*¹ |
6.4%*¹ |
|
3 |
PEG 400 |
30%*² |
30%*² |
30%*² |
|
4 |
Methanol |
3.5 ml |
3.5 ml |
3.5 ml |
|
5 |
Distilled Water |
5 ml |
5 ml |
5 ml |
*¹ = weight of total polymeric solution, *² = weight of polymer
The prepared buccal patches were evaluated for various properties like weight variation, thickness, folding endurance, swelling index, surface pH, moisture uptake, tensile strength, drug content, diffusion study.
EVALUATION OF BUCCAL PATCHES:
Thickness[9]
The thickness of patch was measured by screw gauge with least count 0.001 mm. The thickness uniformity was measured at three different sites and average of three readings was taken with standard deviation.
Weight variation[10]
The three dicks of 1 cm2 was cut and weighed on electronic balance for weight variation test. The test was done to check the uniformity of weight and thus check the batch-to-batch variation.
Moisture uptake[11]
The percent moisture absorption test was carried out to check the physical stability and integrity of the patches at high humid conditions. In the present study the moisture absorption capacities of the patch were determined in the following manner. The patches were placed in the dessicator containing saturated solution of aluminum chloride, keeping the humidity inside the dessicator at 79.5% R. H. After 24 hrs. The patches were taken and weighed the percentage moisture absorption of the patches was found.
Water vapor transmission test [12]
Vapor transmission method was employed for the determination of vapor transmission from the patch. Glass –bottle (length= 5 cm, narrow mouth with internal diameter = 0.8 cm) filled with 2 gm anhydrous calcium chloride and an adhesive (Feviquick®) spread across its rim, was used in the study. The patch was fixed over the adhesive and the assembly was placed in a constant humidity chamber, prepared using saturated solution of ammonium chloride and maintained at 37±2°c. the difference in weigh after 24 hr. was calculated. Vapor transmission rate was obtained as follows:
VTR = ( Amount of moisture transmitted) / (Area × Time)
Tensile Strength [13]:
Satisfactory bioadhesion is essential for successful application of biodhesion drug delivery systems in order to increase the residence time at the site of application and hence to provide the prolonged release of the drug. The tensile strength required to detach the bioadhesive patch from the mucosal surface was applied as a measure of the bioadhesive performance. Several techniques have been reported in literature for measurement of bioadhesive strength. In the present work specially fabricated assembly based on published literature of Gupta et. al was used. Goat buccal mucosa was used as the model surface for bioadhesion testing. After the buccal mucosa was excised and trimmed evenly, it was then washed in phosphate buffer and used immediately.
[adsense:468x15:2204050025]
A) Fabrication of the test assembly –

Fig.1 Developed bioadhesion test apparatus
The working of a double beam physical balance formed the basis of bioadhesion test assembly. The left pan was removed and hung with a stainless steel chain. A glass weighing bottle was hung with the stainless steel chain to balance the weight of the other pan. The height of the total set up was adjusted. Another glass weighing bottle was kept inside the top hung beaker, which was then positioned below the top hung glass bottle. Suitable weights were added (0.5gm) on the right pan to balance the beam of the balance.
B) Method –
This method involves the use of goat buccal membrane as the model mucosal membrane. The fresh slaughter house and it was then washed in phosphate buffer. The two sides of the balance were balanced with a 5gm weight on the right hand side. A piece of fresh membrane was glued to an inverted glass bottle with cyanoacrylate adhesive. The inverted bottle was then stick into the glass beaker by using cyanoacrylate glue, the beaker was then filled with phosphate buffer kept at 37 ± 1°c, such that the buffer just reaches the surface of mucosal membrane, and keeps it moist. This was then kept below the left hand set up of the balance. The test film was glued with the same adhesive to a glass bottle hanging on the left hand side and the balance beam raised with the 5gm weight. This lowered the glass bottle along with the film over the mucosa with a weight of 5gm. The balance was kept in this position for 3 minutes and then slowly water was added to the plastic container in the right pan by burette. The detachment of two surfaces was obtained, weight of water was measured. Then the bioadhesive strength of the film was calculated. Three films were tested on each mucosal membrane. After each measurement, the tissues were gently and thoroughly washed with phosphate buffer and left for 5 minutes before the next experiment. Fresh membrane was used for each batch of films. The tensile strength was calculated by using following formula.
Applied force m × g
Tensile stress (S) = --------------------------------- = --------
Cross sectional area b × t
Where,
S = tensile stress in 980 dyne/cm2
m = mass in grams
g = acceleration due to gravity (980 dyne/cm2)
b = breadth of strips in centimeters
t = thickness of strips in centimeters
Percent elongation at break [13]
The percent elongation at break was measured by formula given below.
Total elongation (L)
Strain = --------------------------------- × 100
Original length (L0)
Where,
L = length after force was applied
L0 = original length
Surface pH [14]
The surface pH of patch was determined in order to investigate the possibility of any irritation on the oral cavity. The patches were allowed to swell for 2 hours in 4 ml distilled water. The surface pH was measured by placing the pH electrode in contact with surface of patch and allowing to equilibrate for 1 minute.
Folding endurance [15]
Folding endurance of the patches was determined by repeatedly folding one patch at the same place till it broke or folded up to 300 times, which is considered satisfactory to reveal good patch properties. The number of times of patch could be folded at the same place without breaking gave the value of the folding endurance.
Drug content [16]
The patch of area 1 cm2 was cut and dissolved in distilled water. The remaining volume was made up with distilled water to 100 ml in 100 ml volumetric flask. Then 1 ml was withdrawn from the solution and diluted to 10 ml. The absorbance of the solution was taken at 233 nm and concentration was calculated. By correcting dilution factor, the drug content was calculated.
Swelling Index[17]
1 cm2 patch of each formulation was accurately weighed placed in a beaker containing 20 ml of water. The weight of each patch (W1) was determined at 5, 15 and 30 minute by pressing the patch with a tissue paper to remove the excess fluid. The swollen patches were then reweighed (W2) and swelling index (SI) was calculated using following formula.
Swelling Index = (W2 – W1) / W1
Where,
W1 is initial weight of the patch and
W2 is weight of the patch after particular time of interval.
Diffusion Study [18]
A modified Franz cell was used for evaluating drug release profile diffusion membrane. The receptor compartment was filled with 25 ml of phosphate buffer pH 7.4 stirred by the use of the Teflon coated bead on a magnetic stirrer. The upper part of the cell has pair of flange between which was placed the cellophane diffusion membrane for release studies. The patch was placed over the diffusion membrane; the flanges held in place were tightened with the screws for the cell setup. The whole assembly was kept on the magnetic stirrer and the temperature was maintained at 37 °c with the water jacket and at 50 rpm speed of magnetic bead. The withdrawal port was covered with the glass cork which prevent air entrapment. The upper portion of the cell is the donor compartment which was open at the top to maintain the exposure of the system to the ambient conditions. The amount of drug permeated into the receptor solution was determined by removing 2 ml of sample at intervals for 6 hrs. The withdrawn volume was replaced with an equal volume of fresh buffer solution. The drug permeated was determined by analyzing the samples at 233 nm. The result of in-vitro released study is represented by following graphs.
Cumulative percentage release versus time.
Stability study[19]
The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors. To assess the drug and formulation stability, stability studies were done according to ICH guideline. The formulated buccal patches were wrapped in aluminum foil and stored at 37 ± 1°c for period of one month. After the period of one month patches were tested for appearance, thickness and drug content.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSION
Three formulations of Metformin HCL buccal patches were formulated using different polymer ratios, the composition of which is shown in table 4. The formulations are subjected to evaluation parameters like thickness, drug content, folding endurance, tensile strength, % elongation, % moisture uptake, IR studies, diffusion studies etc.
PREFORMULATION STUDIES:
Characterization of Metformin hydrochloride
Table 2: Characterization of Metformin hydrochloride
|
TESTS |
SPECIFICATION |
RESULTS |
|
Colour |
White |
Confirms |
|
Physical state |
Powder |
Confirms |
|
Identification |
FTIR |
Positive |
|
Melting point |
224°c- 226°c |
224°c |
|
pH |
6 -7 |
6 |
Characterization of excipients
Characterization of HPMC K 4M
Table 3: characterization of HPMC K4M
|
TESTS |
SPECIFICATION |
RESULT |
|
Colour |
White |
Conform |
|
Odour |
Odourless |
Conform |
|
Physical state |
Powder |
Conform |
|
Identification |
FTIR |
Positive |
|
Solubility |
Soluble in water, insoluble in chloroform, ethanol |
Conform |
Spectroscopic study:
FTIR Study:
A. Spectra 1 : IR of pure drug spectrum
B. Spectra 2 : IR of HPMC K4M
C. Spectra 3 : IR of drug and polymer incompatibility
The FTIR spectrum of metformin hydrochloride is consistent with reference spectra given in analytical profile of drug substance. The drug and polymer were compatible with each other.
Spectra 1: IR of pure drug spectra
Fig. 2: IR spectra of metformin hydrochloride
Spectra 2: IR of polymer:

Fig. 3 : IR spectra of HPMC K4M
Spectra 3: IR of drug and polymer:

Fig. 4: IR spectra of drug and polymer incompatibility
UV spectroscopy
1. Determination of λmax:
The peak showed in the figure is much similar to the reported peak. The spectrum obtained is shown in the figure i.e. 233nm.

Fig.5: UV spectrum of Metformin hydrochloride
2. Preparation of calibration curve for Metformin hydrochloride
The absorbance values obtained, are shown in table. Using concentration and absorbance data, a beer and lambert’s plot was obtained. The plot is given in the figure.
CALIBRATION CURVE:
Calibration Curve of Metformin HCL in Phosphate buffer pH 6.8
Table 4: Calibration Curve in phosphate buffer
|
Sr.No. |
CONCENTRATION |
ABSORBANCE |
|
1 |
5 |
0.5271 |
|
2 |
10 |
0.9957 |
|
3 |
15 |
1.4378 |
|
4 |
20 |
1.9220 |
|
5 |
25 |
2.452 |
|
6 |
30 |
2.9891 |

Fig.6: Calibration graph of Metformin hydrochloride in phosphate buffer
EVALUATION OF BUCCAL PATCHES
The values of thickness, weight variation, % moisture uptake, water vapor transmission, surface pH, folding endurance, tensile strength, % elongation, drug content, swelling index are obtained from the tests is tabulated in the following table.
Table no. 5: Evaluation parameters of buccal patch
|
Parameters |
F1 |
F2 |
F3 |
|
Thickness (mm) |
0.96±0.01 |
0.97±0.01 |
0.97±0.01 |
|
Weight Variation(mg) |
329±1.52 |
331±2.08 |
336±2.38 |
|
% Moisture Uptake |
4.8 |
5.1 |
5.3 |
|
Water Vapor Transmission gm Cm-2h-2 |
3.930×10-6±0.38 |
1.634×10-6±0.22 |
2.216×10-6±0.58 |
|
Surface pH |
7.2 |
6.8 |
6.9 |
|
Folding Endurance |
>300 |
>300 |
>300 |
|
Tensile strength |
2.89gm/mm2 |
3.20gm/mm2 |
3.57gm/mm2 |
|
% Elongation |
08.33% |
16.88% |
33.33% |
|
Drug Content |
96.3 |
95.2 |
98.8 |
|
Swelling index |
25.51 |
24 |
28.15 |
Diffusion study:
Table no.6: Diffusion study
|
Time (min) |
% Release (F1) |
% Release (F2) |
% Release (F3) |
|
0 |
0 |
0 |
0 |
|
30 |
21.33 |
20.44 |
21.88 |
|
60 |
38.68 |
37.55 |
40.9 |
|
90 |
55.65 |
53.81 |
58.71 |
|
120 |
72.95 |
71.36 |
77.65 |
|
150 |
90.27 |
88.43 |
92.22 |
Comparison graph of formulation F1, F2 and F3:
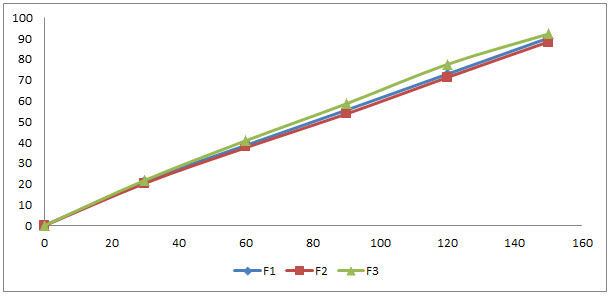
Fig. 7: Comparison graph of diffusion study
Stability study:
The stability study is done for one month and results are given in following table.
Table 7: Stability study
|
FORMULATION CODE |
TIME |
APPEARANCE |
THICKNESS (mm) |
DRUG CONTENT |
|
F1 |
1 month |
Smooth |
0.96 |
90.12 |
|
F2 |
1 month |
Smooth |
0.97 |
87.98 |
|
F3 |
1 month |
Smooth |
0.97 |
92.02 |
CONCLUSION
Medicated patches were evaluated for physical and mechanical properties like weight variation, thickness, swelling study, folding endurance, water vapor transmission test, moisture uptake test, tensile strength, % elongation at break and drug content. All the patches were found to be suitable for formulating in terms of physicochemical characteristics. On the basis of in vitro permeation studies formulation F3 having maximum rate of permeation.
In order to understand mechanism of drug release, in vitro permeation data were treated to kinetic models and linearity was observed. The correlation coefficient obtained from Korsemeyer Peppas as best fit model, r value was found to 0.999 for F2 formulation. According to Design Expert Software formulation F3 was the best formulation having drug content 98.8, tensile strength is 3.57gm/mm² and moisture uptake is 5.3% and also stability study shows that it will be a stable for one month.
From above studies it can be concluded that the polymeric buccal patches of Metformin HCL prepared with different ratios of HPMC K4M.
ACKNOWLEDGEMENT: Authors are grateful to P.D.V.V.P.F’s College of Pharmacy, Ahmednagar to make available the facilities for the present research work and great thanks to my research guide Dr. Chemate Sir.
REFERENCES
REFERENCES:
1. Gupta A, Garg S, Khar RK; Mucoadhesive Buccal Drug Delivery Systems: A Review; Indian Drugs; 1992; 29(13); 586-593
2. D.Harris, J.R. Robinson; Drug delivery via the mucous membrane of oral cavity; J. Pharm. Sci; 1992; 81(1); 1-10
3. JK Pandit, NM Vemuri, SP Wahi, RB Jayachandra; Mucosal dosage form of epidrine hydrochloride using Gantrez-AN 139; The Eastern pharmacist; 1993; 165-170
4. SS Lokhande, SS Lahoti; Buccoadhesive Drug Delivery System: Need; Asian Journal of Biomedical and Pharmaceutical Sciences; 2012; 2(14); 29-36
5. Martindale; The complete drug reference; Kathleen parfit; 32nd edition; Pharmaceutical Press; U. K.; 1999
6. European pharmacopoeia; Council of Europe Strasbourg; 3rd edition; 1997; 1443-1444.
7. Indian pharmacopoeia; 1996; 256-257.
8. Bharath Kumar V, Ashok Kumar A, Sudheer B, Suresh Kumar K, SrinivasaRao V, Kirtinidhi K, Hitesh R Patel and Putta Rajesh Kumar; Formulation design, in vitro evaluation and stability studies on muccoadhesive buccal films of anti- angina calcium channel blocker; journal of Applied Pharmaceutical Science; 2011; 1(6); 136-142.
9. Agarwal V, Mishra B; Design, development, and biopharmaceutical properties of buccoadhesive compacts of pentazocine; Drug Development and Industrial Pharmacy; 1999; 25(6); 701-709.
10. Subhash Deshmane, Madhuri Channawar; Chitosan based sustained release muccoadhesive buccal patches containing Verapamil hudrochloride International journal of pharmacy and pharma science; 2009; 1(s1); 216-230.
11. Y Hu, V Topolkaraev, A. Hiltner, E Baer; Measurement of Water Vapour Transmission Rate in Highly Permeable Films; Journal of Applied Polymer Science; 2001; 81(7); 1624-1633.
12. Agarwal SS, Munjal P; Permeation studies of Atenolol and MetoprololTartarate form three different polymer matrices for transdermal delivery; Ind Journal of Pharma Sci; 2007; 69(4); 535-539.
13. Bottenberg P, Cleymact R, Muynck CD, Remon JP, Coomans D, Michotte Y, Slop D; Development and testing of bioadhesive, fluoride containing slow release tablet for oral use; Journal of Pharmacy and Pharmacology; 1991; 3(7); 457-464.
14. Khanna R, Agarwal SP, Ahuja A; Preparation and evaluation of mucoadhesive buccal films of clotrimazole for oral candida infection; Indian Journal of Pharma Sci. 1997; 59(6); 299-305.
15. Semalty M, Semalty A, Kumar G; Formulation and characterization of muccoadhesive buccal films of Glipizide; Indian Journal of Pharma Science; 2008; 70(1); 43-48.
16. Yehia SA, Gazayerly ON, Basalious EB; Fluconazole muccoadhesive buccal films: in- vitro or in- vivo performance; Current Drug Delivery; 2009; 6(1); 17-27.
17. Bhanja S, Ellaiah P, Chaudhari R, Murthy KV, Panigrahi B, Padhy S. Formulation, development and evaluation of muccoadhesive buccal patches of methotrexate; Journal of Advance Pharma Research; 2010; 1(1); 17-25.
18. Garren K. W and Repta AJ; Buccal Drug Absorption II : In-vitro Diffusion Across the Hamster Cheek Pouch; J. Pharm. Sci.; 1989; 78(2); 160-164.
19. Balamurugan K; Systemic Absorption of Propranolol hydrochloride from Buccoadhesive films; Indian J. Pharm. Sci. 2001; 63(3); 473-480.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









