{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Mayuri Lawar1, Varsha Shende1*, Suvarna Ingle2, Monali Sanghvi1, Naeem Hamdulay1
1Department of Pharmacology, Sinhgad College of Pharmacy, Post-Graduate Research Department, Off Sinhgad road, Vadgaon (Bk), Pune, Maharashtra, India.
2Department of Pharmacology, Indira College of Pharmacy, New Pune Mumbai Highway, Tathwade, Pune, Maharashtra, India.
*shende.varsha@gmail.com
ABSTRACT
Depression and related mood disorders are among the world’s greatest public health problems. Mimosa pudica L. (MP), a creeping perennial herb exhibits analgesic, anxiolytic, diuretic, antiasthmatic, hepatoprotective activities. Although, previous study reveal antidepressant like effects of aqueous extract of MP at behavioral levels, but antidepressant like effects of hydroalcoholic extract of Mimosa pudica L. (HEMP) and its combinational effect with standard antidepressant drugs at behavioral and biochemical level is not scientifically evaluated. Hence, the present investigation evaluates the antidepressant activity of HEMP and its interaction with fluoxetine and imipramine in reserpine-induced behavioural despair in rats and the potential mechanism involved in HEMP action. HEMP at doses 50, 100 and 200 mg/kg p.o., fluoxetine at 5 & 20mg/kg p.o., imipramine at 5 & 15mg/kg p.o. and reserpine 6mg/kg i.p. were administered to different groups of rat (n=6). After reserpine treatment, rats were subjected to forced swimming test followed by assessment of locomotor activity. Rats were sacrificed after behavioural study for estimation of serotonin, monoamine oxidase (MAO) levels in rat brain. Results demonstrate that combination of subeffective doses of HEMP, fluoxetine and imipramine reduced the duration of immobility in forced swimming test, increased serotonin and decreased MAO-A and MAO-B enzyme levels in reserpine treated rats. These findings suggest that combination of HEMP with standard drugs in subeffective doses can exert potential antidepressant like effect through modulation of brain serotonergic system and MAO inhibition.
INTRODUCTION
Depression is one of the most common psychiatric disorders, with a lifetime incidence of up to 20% in the general population, and associated with high morbidity and mortality rate despite major advances in treatment [1]. This has led to a continued search for effective treatments including antidepressant drugs, herbal remedies, psychotherapy and electroconvulsive shock therapy. Recently, the interest in the use of herbal products and the focus on plant research has grown in the western world and developed countries [2]. The majority of currently available psychoactive drugs as herbal remedies are the reflection of such a situation. The demand of herbal remedies as psychotherapeutics is greater and has great growth potential in the global market [3, 4]. Various herbal plants have been used traditionally as antidepressant, amongst them one is MP, which is a well-known weed used as herbal remedy. MP is derived from the word “mimic” means to allude, to sensitivity of leaves and “pudica” means bashful, retiring or shrinking.
MP is a common sensitive, sprawling, and perennial plant and known as ‘Lajvanthi’ or ‘Chuimui’ in local Indian language, which usually grows about 15-45 cm high. Plant bears leaves with dark green colour, feathery, fern-like and divided into one or more pairs of segments near the end of the leaf stalk and flowers are pale pink in fluffy balls [5]. The leaves are bitter, sudorific and tonic and are used in hydrocele, haemorrhoids, fistula, scrofula, conjunctivitis, cuts and wounds and haemorrhages [6, 7]. As a member of family Fabaceae, the root system consists of a taproot and extensive fibrous roots with nodules [8]. The roots are bitter, astringent, acrid, alexipharmic, resolvent, diuretic, antispasmodic, emetic, constipating, and febrifuge and used in vitiated conditions of pitta, leucoderma, vaginopathy, metropathy, ulcers, dysentery, inflammations, burning sensation, haemorrhoids, jaundice, asthma, fistula, small pox, strangury, spasmodic, affections and fevers [6,9].
The flavonoids and tannins from MP are reported for their varied pharmacological activities like, wound healing[10], anti-anxiety[11], antidepressant[12], antiulcer[13], antifertility[14], antibacterial[15], hyperglycaemic[16], anticonvulsant[17] and antihypolipidemic [18]. But till date very few studies are reported on the role of MP in the functional regulation of neurotransmitters and their receptors and antidepressant activity of hydroalcoholic extract of MP (HEMP) is also not revealed yet. Thus in this study we reported the antidepressant effect of HEMP and its interaction with imipramine and fluoxetine on duration of immobility in forced swimming test, brain serotonin, Monoamine oxidase (MAO) enzymes level in reserpine treated rats.
METHODS
Plant material:
HEMP was purchased from local supplier, Prist herbochem, Pune, along with its certificate of analysis and its standardisation was done using thin layer chromatography and preliminary phytochemical screening.
Preliminary phytochemical screening of plant extract:
Organoleptic characteristics, qualitative tests for alkaloids, flavonoids, saponins, steroids, triterpenoids and tannins were performed using standard procedure [19, 20] and quantitative tests were performed for total phenolic and total flavonoid content [21]. Standardisation of extract was done using thin layer chromatography (TLC).
Drugs and solutions:
Fluoxetine hydrochloride (Endoc Pharma, Rajkot), imipramine hydrochloride (Centurion laboratories, Vadodara), reserpine (Loba Cheime, India) and serotonin hydrochloride (Sigma Aldrich, USA). HEMP, Fluoxetine hydrochloride, Imipramine hydrochloride and serotonin hydrochloride were dissolved in distilled water. Reserpine was dissolved in 1% acetic acid solution v/v. All drug solutions were prepared fresh. All the other agents used were of analytical grade.
Experimental animals:
Sprague dawley rats (150- 200 gm) were procured from National Institute of Biosciences, Pune, India. The animals were maintained under standard laboratory conditions at temperature 23 ± 20°C, relative humidity 55 ± 10% and 12:12 h light / dark cycle maintained throughout the experiment. Animals had free access of water and standard laboratory feed ad libitum prior to the dietary manipulation. The animal studies were approved by the Institutional Animal Ethics Committee (Protocol no. SCOP/IAEC/Approval/2011-12/08), constituted for the purpose of control and supervision of experimental animals by Ministry of Environment and Forests, Government of India, New Delhi, India. Animals were naive to drug treatments and experimentation at the beginning of all studies. All tests were conducted between 08:00 and 14:00 h.
Treatments:
Rats were divided into different groups (n=6). Reserpine (6 mg/kg, i.p.) was administered to the rats 24 hrs before activity for induction of depression. Fluoxetine (20mg/kg p.o.), Imipramine (15mg/kg, p.o.) and HEMP (50, 100, 200mg/kg, p.o.) was given 24, 5 and 1 hrs before the behavioural study.
Forced swimming test:
The rats were placed individually in vertical Plexi glass cylinder (50×20 cm) containing water (25°C) at adepth of 30 cm for a 15-min period (pre-test session). At the end of this pretest session, each rat was removed from the water, partially dried with a towel and placed in a warm enclosure, and afterwards returned back to their home cages. Twenty-four hours later, the animals were exposed to the same experimental conditions outlined above for only a 5-min period (test session) and their behavior was video taped with video recorder placed above the cylinder. The total duration of immobility in each rat was measured during the test session. Immobility was assigned when no additional activity was observed other than that required to keep the rat's head above the water. The behavioral assessments were performed by observers blind to experimental treatment. Reserpine and vehicle were administered 24 h before the test session. Imipramine, fluoxetine and different doses of HEMP were administered 24, 5 and 1 h before the test session [22].
Biochemical estimation:
After behavioural test, rats were sacrificed and their brains were rapidly removed on dry ice at -20o C, washed with isotonic saline, weighed and were used for biochemical estimations.
Preparation of Brain tissue:
The brains were thawed, rinsed with isotonic saline, weighed again and homogenized (Homogenizer, REMI, India).The 10% homogenates were prepared in 10 volumes of cold phosphate buffer (10mM, pH 7.4), mingled at 4o C for 20 minutes. Serotonin estimation [23] in hypothalamus and MAO-A and MAO-B enzymes level in whole brain [24] were carried out using spectrophotometer.
Statistical analysis:
Results were expressed as mean ± S.E.M. The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. Statistical significance was considered at P< 0.05 in all the cases.
RESULTS
Effects of fluoxetine on the immobility time in the forced swimming test and on the locomotor activity in the open-field test:
In the present study, one -way ANOVA revealed that fluoxetine (5mg/kg and 20mg/kg p.o.) significantly reduced duration of immobility in rats, when compared with the normal control group (P<0.0001; Fig 1A) and no significant difference was found in locomotor activity in normal control and fluoxetine treated group (Fig 1B).Further Post hoc test found that treatment with fluoxetine (5mg/kg and 20mg/kg p.o.) significantly reduced duration of immobility in rats, when compared with the normal control group (P<0.05 & P<0.001 respectively) in a dose dependent manner.
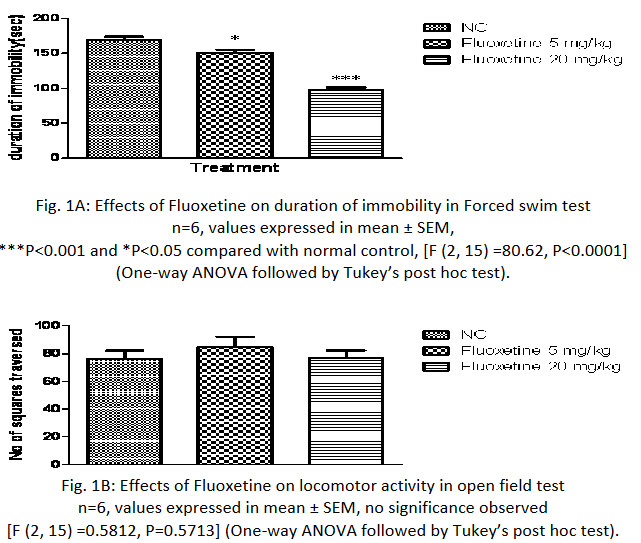
Fig. 1A: Effects of Fluoxetine on duration of immobility in Forced swim test
n=6, values expressed in mean ± SEM,
***P<0.001 and *P<0.05 compared with normal control, [F (2, 15) =80.62, P<0.0001]
(One-way ANOVA followed by Tukey’s post hoc test).
Fig. 1B: Effects of Fluoxetine on locomotor activity in open field test
n=6, values expressed in mean ± SEM, no significance observed
[F (2, 15) =0.5812, P=0.5713] (One-way ANOVA followed by Tukey’s post hoc test).
Effects of imipramine on the immobility time in the forced swimming test and on the locomotor activity in the open-field test:
In the present study, one -way ANOVA revealed that imipramine (5mg/kg and 15 mg/kg p.o.) significantly reduced duration of immobility in rats, when compared with the normal control group (P<0.0001; Fig 2A) and no significant difference was found in locomotor activity in normal control and imipramine treated group (Fig 2B).Further Post hoc test found that treatment with imipramine (5mg/kg and 15 mg/kg p.o.) significantly reduced duration of immobility in rats, when compared with the normal control group (P<0.05 & P<0.001 respectively) in a dose dependent manner.
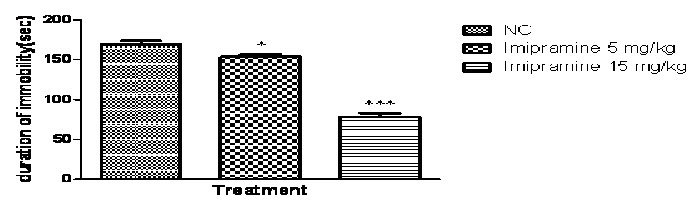
Fig. 2A: Effects of Imipramine on duration of immobility in Forced swim test
n=6, values expressed in mean ± SEM,
***P<0.001 and *P<0.05 compared with normal control, [F (2, 15) =150.4, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
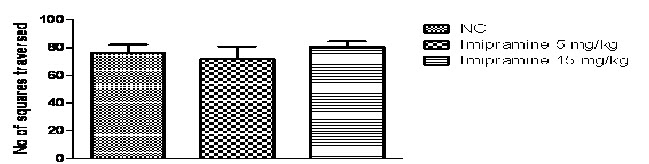
Fig. 2B: Effects of Imipramine on locomotor activity in open field test
n=6, values expressed in mean ± SEM, no significance was found
Effect of HEMP onthe immobility time in the forced swimming test and on the locomotor activity in the open-field test:
In the present study, one -way ANOVA revealed that HEMP at the doses (50, 100 and 200 mg/kg p.o.) significantly reduced duration of immobility in rats, when compared with the normal control (P<0.0001; Fig 3A) and no significant difference was found in locomotor activity in normal control and treated group(Fig 3B).Further Post hoc test found that treatment with HEMP at the doses (50, 100 and 200 mg/kg p.o.)significantly reduced duration of immobility in rats, when compared with the normal control group (P<0.05 & P<0.001 respectively) in a dose dependent manner.
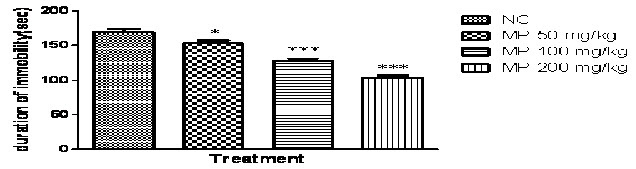
Fig. 3A: Effect of HEMP on duration of immobility in Forced swim test
n=6, values expressed in mean ± SEM,
***P<0.001 and *P<0.05 compared with normal control, [F (3, 20) =49.37, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
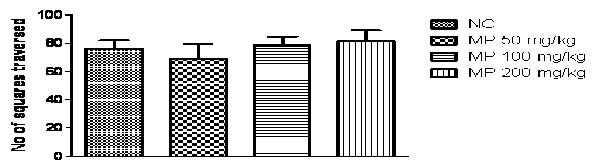
Fig. 3B: Effects of HEMP on locomotor activity in open field test
n=6, values expressed in mean ± SEM, no significance was found
[F (3, 20) =0.5812, P=0.5713] (One-way ANOVA followed by Tukey’s post hoc test).
Effect of Fluoxetine, Imipramine and HEMP on Reserpine induced behavioural despair using forced swim test:
As shown in Fig. 4 one-way ANOVA demonstrates that reserpine (6 mg/kg i.p.) significantly increased duration of immobility in rats when compared with normal control group (P<0.0001). However, treatment with fluoxetine, imipramine and HEMP significantly decreased duration of immobility in rats when compared with reserpine control group.
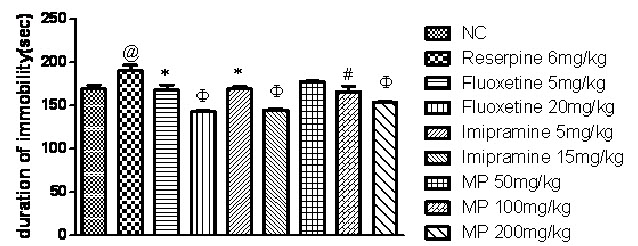
Fig. 4: Effect of Fluoxetine, Imipramine and HEMP on Reserpine induced behavioural despair using forced swim test
n=6, values expressed in mean ± SEM,
@P<0.05 compared with normal control, *P<0.05, #P< 0.01 and ΦP<0.001 compared with reserpine control, [F (8, 45) =12.56, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
Effect of combination of subeffective doses of Fluoxetine and Imipramine with HEMP on Reserpine induced behavioural despair using forced swim test:
As shown in Fig. 5, one-way ANOVA demonstrates that reserpine (6 mg/kg i.p.) significantly increased duration of immobility in rats when compared with normal control group (P<0.05). However, treatment with combination of subeffective doses of fluoxetine (5 mg/kg) and imipramine (5 mg/kg) with HEMP(100 mg/kg) significantly decreased duration of immobility in rats (P<0.001) when compared with reserpine control group.
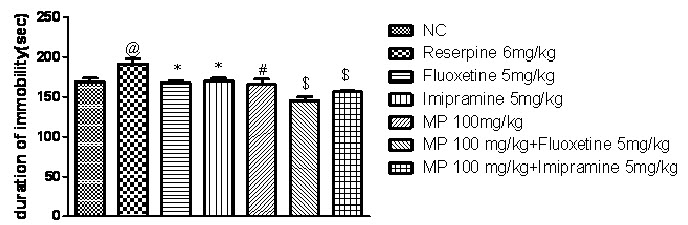
Fig. 5: Effect of combination of subeffective doses of Fluoxetine and Imipramine with HEMP on Reserpine induced behavioural despair using forced swim test
n=6, values expressed in mean ± SEM, @P<0.05 compared with normal control, *P<0.05, #P< 0.01 and P<0.001 compared with reserpine control [F (6, 35) =9.366, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
Effect of combination of subeffective doses of Fluoxetine and Imipramine with HEMPon locomotor activity after reserpine treatment:
Fig. 6, represents reserpine (6 mg/kg i.p.) reduced locomotor activity in rats significantly, when compared with normal control group however, Fluoxetine, Imipramine and HEMP significantly increased locomotor activity in rats in a dose dependent manner, when compared with reserpine control group.
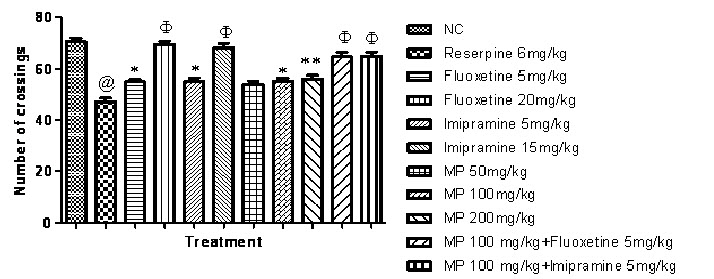
Fig. 6: Effect of HEMP and combination of subeffective doses of Fluoxetine and Imipramine with HEMP on locomotor activity
n=6, values expressed in mean ± SEM,
@P<0.001 compared with normal control, *P<0.05,**P<0.01 and ΦP<0.001 compared with Reserpine control, [F (10, 55) =28.75, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
Effects of HEMP on Serotonin concentration in rat brain:
Fig. 7 represents reserpine (6 mg/kg i.p.) decreased serotonin concentration significantly in rat brain, when compared with the normal control group. While HEMP (200 mg/kg) and combination of subeffective doses of Fluoxetine and Imipramine with HEMP increased serotonin concentration in rat brain hypothalamus, when compared with reserpine control group.
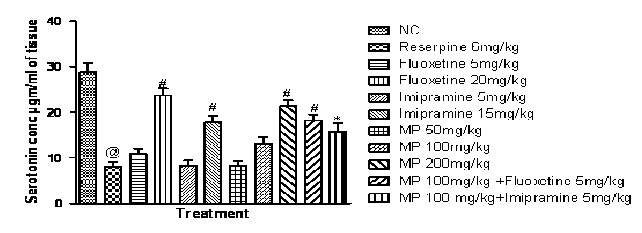
Fig. 7: Effect of HEMP and combination of subeffective doses of Fluoxetine and Imipramine with HEMP on serotonin concentration
n=6, values expressed in mean ± SEM,
@P<0.001 compared with normal control, *P<0.05 and #P<0.001 compared with Reserpine control, [F (10, 55) =24.43, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
Effects of HEMP on MAO enzyme concentration in rat brain:
Fig (8A & B) demonstrates reserpine (6 mg/kg i.p.) increased MAO-A and MAO-B enzyme concentration significantly in rat brain, when compared with the normal control group. While HEMP (100 & 200 mg/kg) and combination of subeffective doses of Fluoxetine and Imipramine with HEMP, decreased MAO-A and MAO-B enzyme concentration in whole rat brain, when compared with reserpine control group.
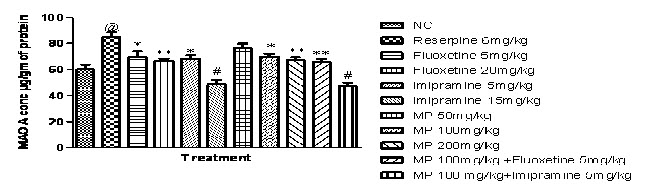
Fig. 8A: Effect of HEMP and combination of subeffective doses of Fluoxetine and Imipramine with HEMP on MAO A concentration.
n=6, values expressed in mean ± SEM,
@P<0.001 compared with normal control, *P<0.05, P<0.001 and #P<0.001 compared with Reserpine control, [F (10, 55) =14.01, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
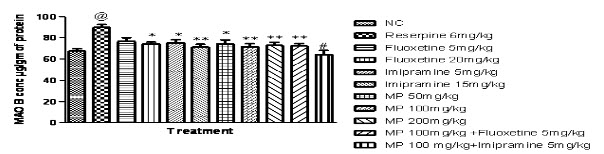
Fig 8B: Effect of HEMP and combination of subeffective doses of Fluoxetine and Imipramine with HEMP on MAO B concentration
n=6, values expressed in mean ± SEM,
@P<0.0001 compared with normal control, *P<0.05, **P<0.001 and #P<0.0001 compared with Reserpine control, [F (10, 55) =4.868, P<0.0001] (One-way ANOVA followed by Tukey’s post hoc test).
DISCUSSION
The present study was designed to evaluate the antidepressant effect of HEMP and its combination with fluoxetine and imipramine in reserpine induced behavioral despair using forced swim test (FST) in rats, which is a widely accepted animal model for evaluation of antidepressant activity in rodents [25,26]. The characteristic behavior scored in these tests is termed as immobility, reflecting behavioral despair as seen in human depression, and it is well known that the antidepressant drugs are able to reduce the immobility time in rodents [25,27]. If antidepressants are administered prior to the tests, the subjects will demonstrate escape-directed behaviors for longer periods of time when compared after vehicle treatment. Thus, the main indication that any given compound has antidepressant like activity is the marked reduction in duration of immobility [28]. Our results showed that fluoxetine (5 and 20mg/kg), imipramine (5 and 15mg/kg) and HEMP (50, 100 and 200mg/kg) possessed antidepressant like activity in the forced swimming test. Further, a compound enhancing locomotor activity may give rise to a false positive effect in the forced swimming test. Therefore, to rule out such possibility the open-field test was carried out parallel with these tests. HEMP did not affect the locomotor activity in the open-field test at the doses that significantly improved antidepressant performance, indicating that antidepressant-like action of HEMP in the forced swimming test is unlikely to be due to an increase in the locomotor activity.
Reserpine a vesicular reuptake blocker is known to produce depression by depletion of biogenic amines in brain [22, 29] and also reported to decrease locomotor activity in rodents [30, 31]. In FST, reserpine (6mg/kg i.p.) significantly potentiated the duration of immobility when compared with normal control rats. Our results showed that pretreatment with fluoxetine (5 and 20mg/kg), imipramine (5 and 15mg/kg) and HEMP (100 and 200mg/kg) possessed antidepressant like activity in the forced swimming test in reserpine treated rats. In addition, combination of subeffective doses of fluoxetine (5 mg/kg) and HEMP (100 mg/kg) and imipramine (5 mg/kg) and HEMP (100 mg/kg) showed significant reduction in immobility time in forced swimming test in reserpine treated rats compared with the effect produced by administration of individual substance. Also, several experiments have shown that reserpine and chlorpromazine are known to decrease spontaneous locomotor activity in rodents [32]. In line with these observations, the present study revealed that reserpine treatment in rodents significantly decreased locomotor activity in open field test compared with vehicle treated group. However, treatment with combination of subeffective doses of fluoxetine (5 mg/kg) and imipramine (5 mg/kg) with HEMP (100 mg/kg) significantly increased locomotor activity in the open-field test in reserpine treated compared to the vehicle treated reserpine rats.
Neurobiological basic research as well as clinical studies has revealed that the monoamines (5-HT, noradrenaline and dopamine) have a crucial role in the development of the depression syndrome. 5-HT may be seen as a crucial ‘fine tuner’ of normal and pathological processes in addition to having a role as a conventional neurotransmitter [33]. As discussed before, reserpine is a vesicular monoamine reuptake blocker and also depletes the monoamine concentration in the rat brain [29]. From the in-vitro studies, it was observed that reserpine treatment lowered the serotonin concentration. Moreover, result of the present study showed that combination of subeffective doses of fluoxetine (5 mg/kg) and imipramine (5mg/kg) with HEMP (100 mg/kg) increased serotonin level in the rat hypothalamus which are in accordance of the previous study. The previous research on MP plant has also suggested the presence of indolamines (serotonin and melatonin) [34] which support the increase in serotonin concentration in rat brain after treatment of HEMP.
Monoamine oxidase (MAO) regulates the metabolic degradation of catecholamines, serotonin and other endogenous amines in CNS [35]. Inhibition of this enzyme causes a reduction in metabolism and subsequent increase in the concentration of biogenic amines. Thus MAO-A and MAO-B enzymes deaminate biogenic amines responsible for depression 35, 36. Treatment with reserpine, deplete levels of monoamines and further, treatment of subeffective doses of fluoxetine (5 mg/kg) and and imipramine (5 mg/kg) with HEMP (100 mg/kg) significantly demonstrated reduction in MAO levels in reserpinised rats. Thus, increase in the serotonin and decrease in the MAO enzymes by HEMP and its combination with fluoxetine and imipramine signifies its antidepressant- like effect. This observed effect of HEMP may be due to modulation of both serotonergic and noradrenergic pathways.
The phytochemical screening of the HEMP revealed presence of flavonoids, alkaloids, saponins, tannins and triterpenoids which may be responsible for its pharmacological activity. However, quantitative study showed the presence of adequate concentrations of total flavonoid and total phenol which may contribute to its antidepressant activity.
CONCLUSION
In conclusion, the present study clearly demonstrates that treatment with HEMP and its combination with fluoxetine and imipramine could significantly decrease the immobility time in the forced swimming test and increased locomotor activity in open field test in reserpine treated rats. These results suggest that HEMP possessed potent antidepressant-like property via serotonergic pathway and modulation of MAO enzyme levels in rat brain. Moreover, further preclinical studies to be performed that will be important to improve population health in general.
REFERENCES
1. Seo MK, Song JC, Lee SJ, Koo KA, Park YK, Lee JG, Park SW and Kime YH. Antidepressant-like effects of Bupleuri Radix extract. European Journal of Integrative Medicine, 2012;4(4):392-399.
2. Loya AM, Gonzalez-Stuart A and Rivera JO. Prevalence of polypharmacy, polyherbacy, nutritional supplements use and potential product interactions among older adults living on the United States-Mexico border: a descriptive, questionnaire-based study. Drugs Aging, 2009; 26(5): 423-436.
3. Dahanukar SA , Kulkarni RA and Rege NN. Pharmacology of medicinal plants and natural products. Indian Journal of Pharmacology, 2000; 32: S81-S118.
4. Detke MJ, Rickels M and Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Pshycopharmacology, 1995;121: 66-72.
5. Annonymous. Common sensitive plant Mimosa pudica, Fact sheet, Land Protection (Invasive Plants and Animals) department of primary industries and fisheries, queensland. Available from: dpi.qld.gov.au/4790_7242.html [2007; cited 8 November 2011.]
6. Pande M and Pathak A. Preliminary pharmacognostic evaluations and phytochemical Studies on roots of Mimosa pudica (lajvanti). Int J Pharm Sci Rev Res., 2010;1(1): 50-52.
7. Sharma PC, Yelne MB and Dennis TT. Database on medicinal plants Govt. of India. 2001; 369-379.
8. Annonymous. The World Health Report. Mental health: new understanding new hope.WHO, Geneva, 2001.
9. Vaidyaratanm PS. Indian Medicinal Plants database Kottakkal. Arya Vidyashala: Orient Longman, 2001; 2.
10. Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC and Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. Journal of Ethnopharmacology, 2009; 124(2): 311-315.
11. Ayissi MR, Gartside S, Bum E, Njikam N, Okello E and McQuade R. Effect of Mimosa pudica (Linn.) extract on anxiety behaviour and GABAergic regulation of 5-HT neuronal activity in the mouse. Journal of Psychopharmacology, 2012; 26(4): 575-583
12. Molina M, Contreras CM and Alcantara P. Mimosa pudica may possess antidepressant actions in the rat. Phytomedicine, 1999; 6(5): 319-323.
13. Kumar BK, Ramesh C, Eienstien J, Girish K and Saleem BS. Evaluation of antiulcer activity of mimosa pudica Ethanolic extract in rats. International Journal of Pharm Sciences, 2011; 2(4);1117-1124.
14. Ganguly M, Devi N, Mahanta R and Borthakur MK. Effect of Mimosa pudica root extract on vaginal estrous and serum hormones for screening of antifertility activity in albino mice. Contraception, 2007; 76(6): 482-485.
15. Muthukumaran P, Padmapriya P, Salomi S, Umamaheshwari R, Kalaiarasan P, and Malarvizhi C. In vitro antimicrobial activity of leaf powder. Asian Journal of Pharmaceutical Research, 2011; 1(4):108-110.
16. Amalraj T and Ignacimuthu S. Hyperglycemic effect of leaves of Mimosa pudica Linn. Fitoterapia, 2002; 73(4): 351-352.
17. Ngo BE, Dawack D L, Schmutz M, Rakotonirina A, Rakotonirina S V, Portet C, Jeker A, Olpe HR and Herrling P. Anticonvulsant activity of Mimosa pudica Decoction. Fitoterapia, 2004; 75(3-4): 309-314.
18. Rajendran R and Krishnakumar E. Hypolipidemic Activity of Chloroform Extract of Mimosa pudica Leaves. Avicenna Journal of Medical Biotechnology, 2010; 2(4): 215-221.
19. Rangari V. “Pharmacognosy and Phytochemistry”, Career publications, Nashik, 2002; 1(1).
20. Khandelwal K. “Practical Pharmacognosy”, Nirali Prakashan, Pune, 2008; 19.
21. McDonald S, Prenzler PD, Autolovich M and Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chemistry, 2001;73(1): 73-84.
22. Bolandghamat S, Moghimi A and Iranshahi M. Effects of ethanolic extract of pine needles (Pinus eldarica Medw.) on reserpine-induced depression-like behavior in male Wistar rats. Pharmacognosy Magazine, 2011; 7(27):248-253.
23. Clark CT, Weissbach H and Udenfriend SJ. The Estimation of 5-hydroxytrptamine (Serotonin) in biological tissues. The Journal of Biological Chemistry, 1954; 210: 337-344.
24. PanY, Kong L, Xia X, Zhang W, Xia Z, Jiang F. Antidepressant-like effect of icariin and its possible mechanism in mice. Pharmacology Biochemistry and Behavior, 2005; 82(4):686-694.
25. Porsolt RD, Anton G, Blavet N and Jalfre M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. European Journal of Pharmacology, 1978; 47: 379-391.
26. Dhir A, Malik S, Kessar SV, Singh KN, Kulkarni SK. Evaluation of antidepressant activity of 1-(7-methoxy- 2-methyl-1,2,3,4-tetrahydro-isoquinolin-4-YL)- cyclohexanol, a β-substituted phenylethylamine in mice. European Neuropsychopharmacology, 2011; 21(9): 704-714.
27. Porsolt RD, Bertin A and Jalfre M. Behavioural despair in rats and mice: Strain differences and the effects of imipramine. European Journal of Pharmacology, 1978; 51(3): 291-294.
28. Yi LT, Li CF, Zhan X, Cui CC, Xiao F, Zhou LP, Xie Y. Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2010; 34(7): 1223–1228.
29. Kandel ER. “Principles of neural science”, McGraw-Hill Companies, New York, 2000;1216–1217.
30. Myron A and Hoffer MD. Effects of reserpine and amphetamine on the development of hyperactivity in maternally deprived rat pups. Psychosomatic Medicine, 1980; 42(5), 513–520.
31. O’Gara BA, Chae H and Latham LB. Modification of leech behavior patterns by reserpine-induced amine depletion. The Journal of Neuroscience, 1991; 11(1), 96-110.
32. Messiha FS. Effect of Amantadine on Chlorpromazine and Reserpine-Induced Behavioral Depression in the Mouse. Neuroscience & Biobehavioral Reviews, 1988; 12(3-4): 219-222.
33. Naughton M, Mulrooney JB and Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Human Psychopharmacology, 2000; 15(6): 397–415.
34. Ramakrishna A, Giridhar P and Ravishankar GA . Indoleamines and calcium channel influence on morphogenesis in in vitro cultures of Mimosa pudica L. Plant Signaling & Behavior, 2009; 4(12): 1136-1141.
35. Schurr A and Livne A. Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components. Biochemical Pharmacology, 1976; 25(10): 1201-1203.
36. Billett EE. Monoamine oxidase (MAO) in human peripheral tissues. Neurotoxicology, 2004; 25(1-2): 139-148.
REFERENCE ID: PHARMATUTOR-ART-2235
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 9 Received On: 13/04/2014; Accepted On: 30/06/2014; Published On: 01/09/2014How to cite this article: M Lawar, V Shende, S Ingle, M Sanghvi, N Hamdulay; Effect of Hydroalcoholic extract of Mimosa Pudica Linn. with Imipramine and Fluoxetine on Reserpine-induced Behavioural Despair in Rats; PharmaTutor; 2014; 2(9); 123-134 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









