About Authors:
Patel Amit, Hala Arshad, Beniwal Prashant
Seth G. L. Bihani S.D. College of Technical Education,
Institute of Pharmaceutical Sciences and Drug Research,
Sri Ganganagar, Rajasthan, INDIA
ABSTRACT:
The HPLC detectors are used to detect the solute present in the eluent comes from the HPLC column. There are various types of detectors available, but Detector selection in HPLC system is most valuable part in the solute detection. After comparison of all detector, find out which is the best detector for our solute analysis? Then Detector selection was carried out. Comparisons of all detectors are done on the basis of the sensitivity of the detector for that particular solute.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1251
1. INTRODUCTION
Several features of HPLC Detectors:
(1) it should response to all compounds in the mixture ( a general detector) or it should response with known sensitivity (a specific detector).
(2) It should not response to mobile phase.
(3) It should give linear response to solute concentration.
(4) It should be unaffected by variation in temperature and flow rate.
(5) It should not contribute to zone spreading. 5
Suitable detectors can be broadly divided into the following two classes:
(a) Bulk property detectors which measure the difference in some physical property of the solute in the mobile phase compared to the mobile phase alone, e.g. refractive index and conductivity detectors. They are generally universal in application but tend to have poor sensitivity and limited range. Such detectors are usually affected by even small changes in the mobile-phase composition which precludes the use of techniques such as gradient elution.
(b) Solute property detectors, e.g. spectrophotometric, fluorescence and electrochemical detectors. These respond to a particular physical or chemical property of the solute, being ideally independent of the mobile phase. In practice, however, complete independence of the mobile phase is rarely achieved, but the signal discrimination is usually sufficient to permit operation with solvent changes, e.g. gradient elution. They generally provide high sensitivity (about 1 in 109 being attainable with UV and fluorescence detectors) and a wide linear response range but, as a consequence of their more selective natures, more than one detector may be required to meet the demands of an analytical problem. Some commercially available detectors have a number of different detection modes built into a single unit, e.g. the Perkin- Elmer '3D' system which combines UV absorption, fluorescence and conductometric detection.
Some of the important characteristics required of a detector are the following.
a) Sensitivity, which is often expressed as the noise equivalent concentration, i.e. the solute concentration, Cn, which produces a signal equal to the detector noise level. The lower the value of Cn for a particular solute, the more sensitive is the detector for that solute.
b) A linear response. The linear range of a detector is the concentration range over which its response is directly proportional to the concentration of solute. Quantitative analysis is more difficult outside the linear range of concentration.
c) Type of response, i.e. whether the detector is universal or selective. A universal detector will sense all the constituents of the sample, whereas a selective one will only respond to certain components. Although the response of the detector will not be independent of the operating conditions, e.g. column temperature or flow rate, it is advantageous if the response does not change too much when there are small changes of these conditions.8
2. CLASSIFICATION OF HPLC DETECTORS
-
UV/VIS
- Fixed Wavelength
- Variable Wavelength
- Diode array
-
Refractive index
-Deflection Detector
-Refractive Detector (Fresnel refractometer)
- Fluorescence Detector
- Electrochemical Detector
- Evaporative light scattering
- Conductivity Detector
- Mass detector
- IR detector
2.1 Ultraviolet/visible spectroscopic detectors
Principles
Any chemical compound could interact with the electromagnetic field. Beam of the electromagnetic radiation passed through the detector flow-cell will experience some change in its intensity due to this interaction. Measurement of this changes is the basis of the most optical HPLC detectors.
Radiation absorbance depends on the radiation wavelength and the functional groups of the chemical compound. Electromagnetic field depending on its energy (frequency) can interact with electrons causing their excitation and transfer onto the higher energetical level, or it can excite molecular bonds causing their vibration or rotation of the functional group. The intensity of the beam which energy corresponds to the possible transitions will decrease while it is passing through the flow-cell. According to the Lambert-Bear law absorbance of the radiation is proportional to the compound concentration in the cell and the length of the cell.
The electromagnetic spectrum is traditionally divided into several regions:
|
infrared (IR) |
2,500 - 50,000 nm |
|
near infrared |
800 - 2,500 nm |
|
Visible |
400 - 800 nm |
|
ultraviolet (UV) |
190 - 400 nm |
Three major regions (IR, visible, and UV) are used in the spectroscopy. In liquid chromatography, IR spectrophotometers have found only limited use. There are few transparent polar liquids which can be used as the mobile phase. On the other hand, spectrophotometers working in the range (200 - 600 nm) are used widely as LC detectors.
UV and visible region of the electromagnetic radiation corresponds to the excitation of the relatively low energy electrons such as pi-electrons, or non-paired electrons of some functional groups. For example, n-alkanes could absorb in the UV region below 180 nm. s-electrons require high energy radiation to get excited and to show absorption of the radiation. But any compounds which have benzene ring will show absorbance at 205-225 and 245-265 nm. The last corresponds to the excitation of conjugated p-electrons of the benzene ring.
The majority of organic compounds can be analyzed by UV/VIS detectors. Almost 70% of published HPLC analyses were performed with UV/VIS detectors. This fact, plus the relative ease of its operation, makes the UV detector the most useful and the most widely used LC detector. 4
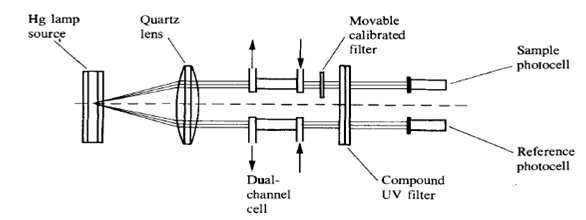
2.1.1 Fixed wavelength detectors
HPLC detectors which does not allow to change the wavelength of the radiation called fixed-wavelength detectors. They are usually very cheap, in the nowadays you probably wont be able to find that type of the detector on the market.
low-pressure mercury vapor lamp emit very intense light at 253.7 nm. By filtering out all other emitted wavelengths, manufacturers have been able to utilize this 254 nm line to provide stable, highly sensitive detectors capable of measuring subnanogram quantities of any components which contains aromatic ring. The 254 nm was chosen since the most intense line of mercury lamp is 254 nm, and most of UV absorbing compounds have some absorbance at 254 nm.
2.1.2 Diode Array Detector
It is also a UV detector.Light from the broad emission source is collimated by an achromatic lens system so that the total light passes through the detector cell onto a holographic grating. In this way the sample is subjected to light of all wavelengths generated by the lamp. The dispersed light from the grating is allowed to fall onto a diode array. The array may contain many hundreds of diodes and the output from each diode is regularly sampled by a computer and stored on a hard disc. At the end of the run, the output from any diode can be selected and a chromatogram produced using the UV wavelength that was falling on that particular diode. During chromatographic development, the output of one diode is recorded in real time producing a real time chromatogram. It is seen that by noting the time of a particular peak, a spectrum can be obtained by recalling from memory the output of all the diodes at that particular time.10
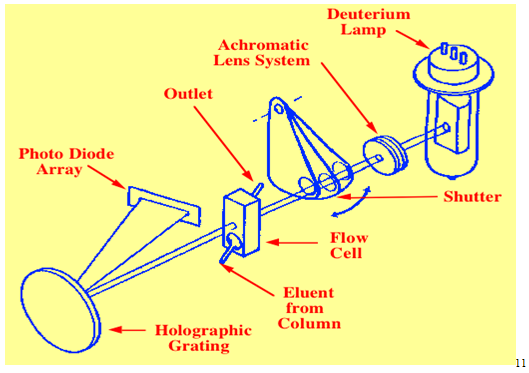
Advantages : Various advantages are, namely :
(a) A very selective detector which will detect only such solutes that specifically absorb UV/visible radiation e.g., alkenes, aromatics and compounds having multiple bonds between C, O, N and S.
(b) The mobile-phase* employed ideally must not absorb any radiation.
2.2 Refractive index detectors:
These bulk property detectors are based on the change of refractive index of the eluant from the column with respect to pure mobile phase. Although they are widely used, the refractive index detectors suffer from several disadvantages - lack of high sensitivity, lack of suitability for gradient elution, and the need for strict temperature control (±0.001 °C) to operate at their highest sensitivity. A pulseless pump, or a reciprocating pump equipped with a pulse dampener, must also be employed. The effect of these limitations may to some extent be overcome by the use of differential systems in which the column eluant is compared with a reference flow of pure mobile phase. The two chief types of RI detector are as follows.
2.2.1 The deflection refractometer, which measures the deflection of a beam of monochromatic light by a double prism in which the reference and sample cells are separated by a diagonal glass divide. When both cells contain solvent of the same composition, no deflection of the light beam occurs; if, however, the composition of the column mobile phase is changed because of the presence of a solute, then the altered refractive index causes the beam to be deflected. The magnitude of this deflection is dependent on the concentration of the solute in the mobile phase.
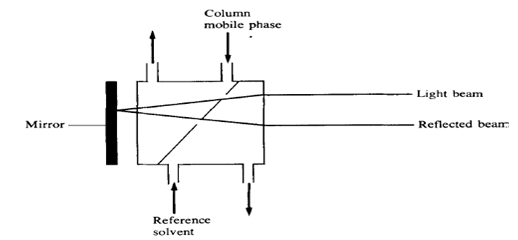
2.2.2 The Fresnel refractometer which measures the change in the fractions of reflected and transmitted light at a glass-liquid interface as the refractive index of the liquid changes. In this detector both the column mobile phase and a reference flow of solvent are passed through small cells on the back surface of a prism. When the two liquids are identical there is no difference between the two beams reaching the photocell, but when the mobile phase containing solute passes through the cell there is a change in the amount of light transmitted to the photocell, and a signal is produced. The smaller cell volume (about 3 ilL) in this detector makes it more suitable for high-efficiency columns but, for sensitive operation, the cell windows must be kept scrupulously clean.8
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.3 Fluorescence detectors
compounds (solutes) present in the mobile-phase on being passed as column effluent through a cell irradiated with Xenon or Deuterium source first absorb UV radiation and subsequently emit radiation of a longer wavelength in two different manners, namely
(a) Instantly-termed as ‘Fluorescence,
(b) After a time-gap-known as ‘Phosphorescence6
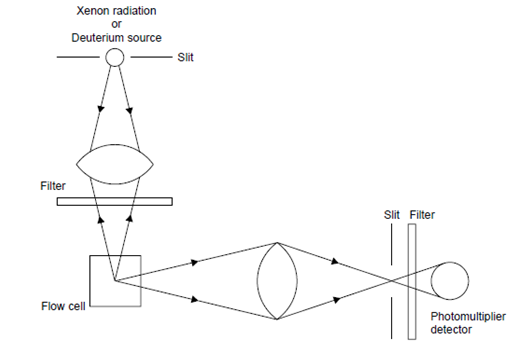
When a molecule adsorbs light, a transition to a higher electronic state takes place and this absorption is highly specific for the molecules concerned; radiation of a specific wavelength or energy is only absorbed by a particular molecular structure. If electrons are raised to an upper excited single state, due to absorption of light energy, and the excess energy is not immediately dissipated by collision with other molecules or by other means, light will be emitted at a lower frequency as the electron returns to its ground state and the substance is said to fluoresce. As some energy is always lost before emission occurs then, in contrast to Raman scattering, the wavelength of the fluorescent light is always greater than the incident light. Detection techniques based on fluorescence affords greater sensitivity to sample concentration, but less sensitivity to instrument instability, (e.g. sensor temperature and pressure). This is due to the fluorescent light being measured against a very low light background (i.e., against a very low noise level). This is opposite to light absorption measurements, where the signal is superimposed on a strong background signal carrying a high noise level.11
These devices enable fluorescent compounds (solutes)present in the mobile phase to be detected by passing the column effluent through a cell irradiated with ultraviolet light and measuring any resultant fluorescent radiation. Although only a small proportion of inorganic and organic compounds are naturally fluorescent, many biologically active compounds (e.g. drugs) and environmental contaminants (e.g. polycyclic aromatic hydrocarbons) are fluorescent and this, together with the high sensitivity of these detectors, explains their widespread use. Because both the excitation wavelength and the detected wavelength can be varied, the detector can be made selective. The application of fluorescence detectors has been extended by means of pre- and post-column derivatisation of non-fluorescent or weakly fluorescing compounds.8
Derivatisation for fluorescence detectors is based on the reaction of nonfluorescent reagent molecules (fluorotags) with solutes to form fluorescent derivatives; the reagent dansyl chloride (I) is used to obtain fluorescent derivatives of proteins, amines and phenolic compounds, the excitation and emission wavelengths being 335-365 nm and 520 nm, respectively.
2.4 Electrochemical detectors
The term 'electrochemical detector' in HPLC normally refers to amperometric or coulometric detectors, which measure the current associated with the oxidation or reduction of solutes. In practice it is difficult to use electrochemical reduction as a means of detection in HPLC because of the serious interference (large background current) caused by reduction of oxygen in the mobile phase. Complete removal of oxygen is difficult so that electrochemical detection is usually based on oxidation of the solute.
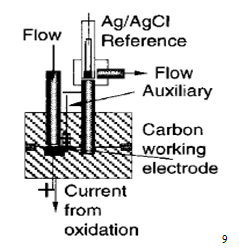
Examples of compounds which can be conveniently detected in this way are phenols, aromatic amines, heterocyclic nitrogen compounds, ketones, and aldehydes. Since not all compounds undergo electrochemical oxidation, such detectors are selective and selectivity may be further increased by adjusting the potential applied to the detector to discriminate between different electro active species. It may be noted here that an anode becomes a stronger oxidising agent as its electrode potential becomes more positive. Of course, electrochemical detection requires the use of conducting mobile phases, e.g. containing inorganic salts or mixtures of water with water-miscible organic solvents, but such conditions are often difficult to apply to techniques other than reverse phase and ion exchange chromatography.8 In short, the amperometric detector is presently considered to be the best electrochemical detector having the following distinct advantages, such as :
(i) very small internal cell-volume,
(ii) high degree of sensitivity,
(iii) more limited range of applications, and
(iv) excellent for trace analyses as UV-detector lacks adequate sensitivity6
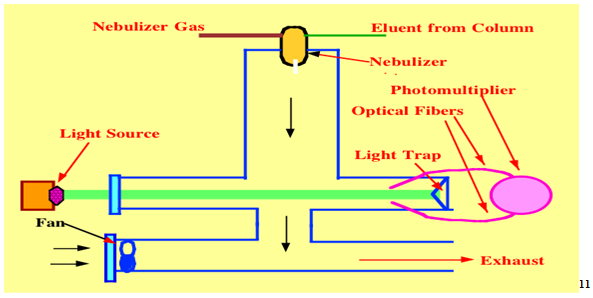
2.5 Evaporative light scattering
Detection is based on the scattering of a beam of light by particles of compound remaining after evaporation of the mobile phase. this detector is of growing importance; it is a universal detector and does not required a compound to have a chromophore for detection. Application include the analysis of surfactant, lipid, and sugar. Unlike the refractive index detector ,which was formerly used for this analysis , it can be with gradient elution and is robust enough to function under wide range of operating conditions. however, it can not be used with involatile material such as buffer in mobile phase or to detect very volatile analytes . typical application include : analysis of chloride and sodium ions in pharmaceuticals, lipids used as component in formulations, sugar and sugar polymers. Sensitive to ca 10 ng analyte.9
2.6 The Electrical Conductivity Detector
The electrical conductivity detector measures the conductivity of the mobile phase. There is usually background conductivity which must be backed-off by suitable electronic adjustments. If the mobile phase contains buffers, the detector gives a base signal that completely overwhelms that from any solute usually making detection impossible. Thus, the electrical conductivity detector is a bulk property detector and will sense all ions, whether they are from a solute, or from the mobile phase. In order to prevent polarization of the sensing electrodes, AC voltages must be used and so it is the impedance not the resistance of the electrode system that is actually measured. From a physical chemistry stand point the conductivity of a solution is more important than its resistance. However, it is the resistance (impedance) of the electrode system that determines the current across it.11
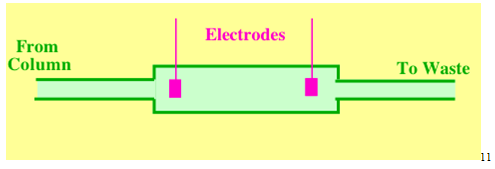
The electrical conductivity detector has a sensitivity (minimum detectable concentration) of about 5 x 10-9 g/ml and a linear dynamic range of about 200 where 0.97 < r < 1.03. It is used in probably over 95% of all analyses involving ion exchange procedures to separate inorganic and organic ions.
2.7 Mass detector
The mass spectrometer is a very sensitive and selective instrument. However, the introduction of the eluent into the vacuum chamber and the resulting significant pressure drop reduces the sensitivity. The gas exhaust power of a normal vacuum pump is some 10 ml min-' so high capacity or turbo vacuum pumps are usually needed. The gas-phase volume corresponding to 1 ml of liquid is 176 ml for n-hexane, 384 ml for ethanol, 429 ml for acetonitrile, 554 ml for methanol, and 1245 ml for water under standard conditions (O'C, 1 atmosphere). The elimination of the mobile phase solvent is therefore important, otherwise the expanding eluent will destroy the vacuum in the detector. Several methods to accomplish this have been developed. The commercialized interfaces are thermo-spray, moving-belt, electrospray ionization, ion-spray, and atmospheric pressure ionization. The influence of the eluent is very complex, and the modification of eluent components and the selection of an interface are therefore important. Micro-liquid chromatography is suitable for this detector, due to its very small flow rate (usually only 10 pl min-I).3
2.8 Infrared detector
Two type of infrared absorbance detector are available. The wavelength scaning is provided by semicircular filter wedges, the wavelength range being from 4000-690 cm-1 . the other fourier transform type is more sophisticated and manufacturer provides the accessories for use as HPLC detector. Cells or Windows are made up of NaCl or CaF2.
It is not very sensitive and have serious drawback that most mobile phase solvents absorb strongly in the IR region.1
3. SPECIFICITY OF DETECTORS.3
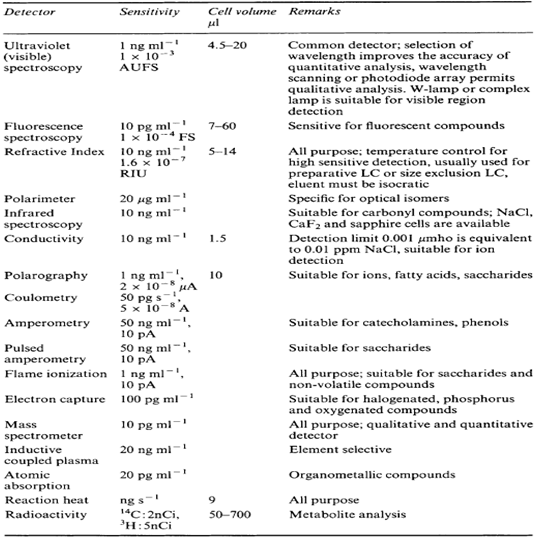
4. CONCLUSION.:
From the study of HPLC detectors, It is concluded that the different detectors having its own characteristics, but its use depend on the sensitivity of detector. For example: mass detector having good sensitivity. That’s why it is used to detect minor quantity of the solute in the sample. But selection of detector is also depending on the characteristics of the solute to be detected. Most widely used detector in HPLC is UV-visible detector (variable wavelength detector.)
REFERENCES
1. Beckett A.H., Stenlake J.B. Practical Pharmaceutical chemistry. 4th edition Part two. Page No:160-161.
2. Connors K.A. A textbook of pharmaceutical analysis. Third edition. New York: A Wiley-interscience publication. page no: 412-414.
3. Hanai T., HPLC A Practical Guide.Published by The Royal Society of Chemistry Cambridge. page no: 18-22.
4.hplc.chem.shu.edu/NEW/HPLC_Book/Detectors/det_uv.html.Accessed on 20/07/2011
5. Kamboi P.C., Pharmaceutical analysis instrumental methods. first edition 2010. Delhi: vallabh publication. page no: 257-265.
6. Kar Ashutosh. Pharmaceutical Drug Analysis. 2nd edition. New Delhi. New Age International Publication. Page no:-461-462.
7. Kealey D., Haines P.J. Analytical Chemistry. 2nd edition. Viva Book Pvt. Ltd. Page no: 162-165.
8. Mendham J.,Denny R.C.,Barnes J.D.,Thomas M. Vogel’s textbook of Quantitative chemical analysis. sixth edition. published by Dorling Kindersley pvt.ltd. page no: 305-310.
9. Watson D .G. pharmaceutical analysis. second edition. published by Elsevier page no: 281-286.
10. Willard H.H.,Dean J.A., Merritt L.L.,Settle F.A. Instrumental Method Of Analysis. First Indian edition 1986. New Delhi. CBS Publication and Distributor. Page no:-600-606.
11. library4science.com;Accessed on 06/08/2011.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









