{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Hemendrasinh J. Rathod*, Dhruti P. Mehta, Jitendra singh Yadav
Department of Pharmaceutics,
Vidyabharti Trust College of Pharmacy,
Umrakh, Gujarat, India.
* hariomh.j.rathod@gmail.com
ABSTRACT
The purpose of writing the review on gastroretentive drug delivery systems (GRDDS) was to accumulate the current literature with a special emphasis on several gastroretentive approaches that have recently become important methodologies in the field of site-specific orally administered sustained/controlled release drug delivery. Technological efforts have been made in research and development of rate-controlled oral drug delivery systems to solve physiological difficulties, like short gastric residence times (GRT) and unpredictable gastric emptying times (GET). GRDDS are an approach to prolong the GRT, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effect. Conventional oral dosage forms pose low bioavailability problems because of their quick gastric transition from the stomach, particularly in case of drugs that are less soluble at an alkaline pH of the intestine. Also, drugs that produce their local action in the stomach get quickly emptied and don’t get sufficient residence time in the stomach. Several efforts have been made to extend the retention time of drug delivery system to reduce the frequency of dose administration. GRDFs not only prolong dosing intervals, but also increase patient compliance beyond the level of existing controlled release dosage forms. This article gives an overview on advantages, disadvantages and characterization of gastroretentive drug delivery systems. This review also includes commercially available gastroretentive products and patents.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2419
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 7 Received On: 08/02/2016; Accepted On: 29/02/2016; Published On: 01/07/2016 How to cite this article: Rathod HJ, Mehta DP, Yadav JS; A review on Gastroretentive Drug Delivery Systems; PharmaTutor; 2016; 4(7); 29-40 |
INTRODUCTION [1-5]
The oral delivery of drugs is the most favoured route of administration because of ease of administration. Drug bioavailability of oral dosage forms is subjective by various factors. One of the significant factor is a gastric residence time (GRT) of these dosage forms. Truly, gastric retention has received important interest in the past few years as many of the conventional oral delivery systems have some limits related to fast gastric emptying time. Gastroretentive dosage form is a type of novel drug delivery system which can persist in the stomach for prolonged period of time and thus increases the GRT of drugs. Gastro-retention helps to improve bioavailability of drugs.
The classification of different modes of gastric retention:
- High-density (Sinking) systems
- Low-density (Floating) systems
- Expandable systems
- Superporous hydrogel systems
- Mucoadhesive systems
- Magnetic systems
The conventional drug delivery system achieves and also maintained the drug concentration in the therapeutically effective range desired for treatment, only when taken numerous times a day. A drug that has a narrow absorption window in the GIT may have poor absorption. For these drugs, GRDDS offer the advantages in extending the gastric emptying time.
Many problems are faced in preparing controlled release systems for better absorption and improved bioavailability. Drug absorption from the GIT is a complex process and is subject to several variables. It is broadly recognized that the extent of GIT drug absorption is correlated to contact time with small intestinal mucosa. GRDDS can persist in the GI region for many hours and therefore significantly extend the GRT of drugs. Extended gastric retention increases bioavailability, decreases drug waste and increases solubility of drugs which are less soluble in high pH environment. To prepare a successful stomach specific or a gastroretentive DDS, numerous techniques are presently used like hydrodynamically balanced systems (HBS)/ floating drug delivery system, low density system, raft systems incorporating alginate gels, mucoadhesive or bioadhesive systems, high density systems, super porous hydrogelsand magnetic systems. Current progress in technology has provided feasible dosage alternatives which can administered by different routes of administration like oral, topical, parenteral, rectal, nasal, ocular, vaginal, etc. But out of all these routes, oral route is considered as the best preferred and practiced way of drug delivery, due to the following reasons:
- Ease of administration
- Ease of production
- More flexibility in designing
- Low cost
Many drugs given by oral route are subjected to absorption through the GIT, with major absorption from the stomach and intestine. Drugs, which is absorbed from the stomach or show local effect, should spend extreme time in stomach. This is found very hard to happen, in case of the conventional dosage forms such as capsules and tablets, due to the gastric emptying. Gastric emptying of dosage form depends on several factors like temperature and viscosity of the meal, volume and composition of the meal, emotional state of the individual, the pH of the stomach, body posture, etc. Prolonged gastric retention of the drug is required in the following conditions:
- The drug is best absorbed from the stomach. E.g., Aspirin, Phenylbutazone, etc.
- Slow dissolving drugs.
- Dissolution and absorption of drug is stimulated by the food. E.g., Griseofulvin.
- Drug show local effects within the stomach.
- Gastric fluids facilitate and increase the disintegration and dissolution of the drug.
In order to achieve all these situations, various methods of the controlled drug delivery have been established. One of these types of the methods, which ensure that a particular drug/dosage form remains in the stomach for longer duration of time, is GRDDS.
However, in following condition gastroretention is considered undesirable:
- For gastro irritant drugs.
E.g., Diclofenac sodium, Ibuprofen, Acetylsalicylic acid, etc.
- For acid labile drugs that are stable at gastric pH.
E.g., Macrolide antibiotics
- Drugs which get absorbed throughout the GIT equally.
STOMACH -AN OVERVIEW [6-7]
The stomach is J shaped enlargement of GIT directly inferior to the diaphragm in epigastric, umbilical and left hypochondriac regions of the abdomen. It connects esophagus to the duodenum, the first part of the small intestine and provides a barrier to the delivery of drugs to the small intestine.
Anatomy of stomach:
The stomach has four regions:
(1) Cardia (2) Fundus (3) Body (4) Pylorus

Figure 1. Anterior view of regions of stomach [6]
Gastric emptying:
The process of gastric emptying happens both during fasting and fed state. In the fasted state, it is categorized by an interdigestive cycle both through the stomach and small intestine, every 2-3 hours. This activity is called the interdigestive myoelectric cycle or migrating myoelectric complex (MMC). It is composed of four phases (Figure 2).
|
Phase |
Duration |
|
Phase-I (Basal phase) |
30-60 min with infrequent contractions |
|
Phase-II (Preburst phase) |
20-40 min with the irregular action potential and contractions as the phase developments, the intensity and the frequency also rises gradually |
|
Phase-III (Burst phase) |
10-20 min, it contains intense and regular contractions for short periods. It’s due to this wave that all the undigested material is swept from stomach to the small intestine |
|
Phase-IV |
0-5 min and happens between phase three & one of two successive cycles |

Figure 2. Gastrointestinal motility patterns [7]
After the digestion of mixed meal, the pattern of contractions changes from fasted to fed state. This is also recognized as digestive motility pattern and contains endless contractions as in phase II of fasted state. The contractions result in decreasing the size of food particles (<1 mm), which are propelled towards the pylorus in the suspension form. Throughout the fed state onset of mmc is postponed resulting in a slowdown of the gastric emptying rate. Scintigraphic studies including the measurements of the gastric emptying rate in healthy human subjects have discovered that an orally administered controlled release dosage form is primarily subject to two physiological difficulties:
1. Short GRT
2. Unpredictable gastric emptying rate
Yet additional major difficulty encountered through the oral route is first pass effect that leads to decreased systemic bioavailability of numerous drugs.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
GASTRORETENTIVE DRUG DELIVERY SYSTEMS [8]
Drugs which are easily absorbed from the gastrointestinal tract and those with short half-lives are quickly eliminated from the systemic circulation due to which frequent dosing is required. To overcome this problem, gastroretentive drug delivery systems which provide effective plasma drug concentration for longer periods thereby reducing the dosing frequency are being formulated. It also has an advantage of minimizing the fluctuations in plasma drug concentration by delivering the drug in a controlled and reproducible manner. If the drugs are poorly soluble in intestine because of alkaline pH, gastric retention may improve the solubility before they emptied resulting in GI absorption of drugs with narrow therapeutic absorption window and also monitoring the release of drugs that have site specific-absorption limitation. Drugs that might take benefit of gastric retention contain the drugs whose solubility is fewer in the higher pH of the small intestine than stomach (E.g., Cinnarizine, Chlordiazepoxide, etc.), the drugs prone to degradation in intestinal pH (E.g., Captopril), and drugs for local action in stomach (E.g., Misoprostol). Sedatives, antibiotics, catecholamines, anticonvulsants, analgesics, anti-hypertensives, vitamins and muscle relaxants can also be administered in hydrodynamically balanced systems (HBS) dosage form (Figure 3).
Gastroretentive drug delivery systems extend the dosing intervals and therefore improve patient compliance. The presence of drug in solution form is an important requisite for the drug to get absorbed. But, if the drug solubility is poor, the time required for drug to dissolve within stomach will be high and transit time becomes most severe factor, which might consequently affect the absorption of the drug. So, dose of administration of such drugs should be kept at repeated intervals in a single day. However, other formulations/novel dosage forms like liposome, nanoparticle, microspheres, etc. can also use for controlled release effect, but GRDDS is considered more desirable alternative for improved absorption through the stomach.

Figure 3.Techniques of GRDDS [7]
FACTORS AFFECTING GASTRIC RETENTION [9, 10]
1. Density:Gastric retention time (GRT) is a function of dosage form buoyancy which is dependent on the density. The density of the dosage form must be lower than the gastric contents (1.004 gm/ml).
2. Size:Dosage form units having a diameter of greater than 7.50 mm are stated to have an improved GRT related with those having a diameter of 9.90 mm.
3. Shape of the dosage form:Tetrahedron and ring shaped devices having a flexural modulus of 48 and 22.50 kilo pounds per square inch are reported to have a better GRT at 24 hours compared with other shapes.
4. Single or Multiple unit formulation: Multiple unit formulations show a more expectable release profile and insignificant damaging of performance because of failure of units, allow co-administration of units that have dissimilar release profiles related with single unit dosage forms.
5. Fed/Unfed state:In fasting conditions, gastrointestinal motility is categorized by periods of strong motor activity that occurs every 1.5 to 2 h and if timing of administration of the formulation overlaps with that of the MMC, the gastric retention time of unit can be anticipated to be very short. However, in fed state, MMC is postponed and gastric retention time is significantly longer.
6. Nature of meal: Feeding of fatty acid salts or indigestible polymers can modify the motility pattern of stomach to a fed state, hence reducing the gastric emptying rate.
7. Caloric content:GRT can be improved by 4 to 10 h with a meal which is high in proteins and fats.
8. Age: Elderly people, mostly those over 70 years, have a significantly longer gastric retention time.
9. Frequency of feed: Gastric retention time can rise by over 400 minutes, when consecutive meals are given related with a single meal because of the low frequency of MMC.
10. Gender: Mean ambulatory gastric retention time in males (3.4 ± 0.6 hours) is less correlated with their age and race matched female counterparts (4.6 ± 1.2 hours), regardless of the weight, body surface and height.
11. Posture: Gastric retention time can be differing between supine and upright ambulatory states of patients.
12. Concomitant drug administration: Anticholinergics like atropine and propentheline increase the GRT. Metoclopramide and Cisapride decrease GRT.
13. Disease state: Gastric ulcer, diabetes and hypothyroidism increase the GRT. Hyperthyroidism and duodenal ulcers decrease the GRT.
ADVANTAGES OF GASTRORETENTIVE DRUG DELIVERY SYSTEMS [11, 12]
- Maintenance of constant therapeutic level over longer period of time.
E.g. Beta lactams antibiotics.
- Enhanced bioavailability of drugs.
E.g. Enhancement of bioavailability of controlled release gastroretentive dosage forms (CR-GRDF) of riboflavin in comparison of non CR-GRDF polymeric formulation.
- Gastroretentive dosage form improves patient compliance by decreasing dosing frequency.
- Minimizing mucosal irritation of drugs, by releasing drug slowly at a controlled rate.
E.g. NSAIDs
- Treatment of GI disorders like GERD, Helicobacter pylori infection, etc.
- Floating drug delivery system is a feasible approach for the drugs that have limited absorption in the intestine.
- The floating drug delivery system can reduce the counter activity of body, leading to higher drug efficiency.
- For drugs that have comparatively short half-life, sustained release may result in a flip-flop pharmacokinetics.
- The floating drug delivery systems are beneficial for drugs that are absorbed through stomach.
E.g. Antacids, Ferrous salts, etc.
- Sustained release drug delivery system reduces dosing frequency of drugs with short half-life.
- Bioavailability enhances despite the first pass effect as a result of variations in plasma drug concentration are escaped; a required plasma drug concentration is retained by the continuous drug release.
- Controlled drug delivery of drugs.
DISADVANTAGES OR LIMITATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEMS [11]
- Drugs, that undergo significant first pass metabolism, are not desirable candidate.
- Drugs having solubility or stability problems in the highly acidic gastric environment cannot be formulated as GRDDS.
- For swellable systems, the dosage forms must maintain size larger than the aperture of the resting pylorus for the required time period.
- These systems do not offer important advantages over the conventional dosage forms of drugs, which are absorbed throughout the gastrointestinal tract.
- Some drugs cause irritation to the gastric mucosa.
- The dosage form must be taken with a full glass of water.
- Violent gas generation, disintegration of dosage forms, burst release, dose dumping, and alkaline microenvironment are the limitation for floating drug delivery.
- Patients cannot be dosed these formulations just before going to bed.
- It is effective only when the fluid level in the stomach is sufficiently high.
- However, as the stomach empties and the dosage form is at the pylorus, the buoyancy of the dosage form may be impeded.
- The major challenge for a bioadhesive system is the high turnover rate of gastric mucus. There is also the possibility of esophageal binding with bioadhesive drug delivery systems. The Hydrogel based swelling system takes longer time to swell.
CRITERIA FOR SELECTION OF DRUG CANDIDATE FOR GRDDS [11]
The gastro retentive drug delivery systems are suitable for following types of drug therapy:
- Absorption from upper GIT: Drugs have a particular site for maximum absorption.
Example: Ciprofloxacin
- Drug having low pKa, which remains unionized in stomach for better absorption.
- Drugs having a reduced solubility at higher pH.
Example: Captopril
- Local action as it is seen in the treatment of H. pylori by Amoxicillin trihydrate as in case of ulcers. The bioavailability of drugs that get degraded in alkaline pH can be increased by formulating gastro retentive dosage forms.
Example: Doxifluridine; which degrades in the small intestine.
- To minimize gastric irritation this may be caused by the sudden increase of drug concentration in the stomach.
Example: NSAIDs
- Drugs that act locally in the stomach.
Example: Antacids, Antibiotics for bacterially based ulcers
- Drugs that are absorbed primarily in the stomach.
Example: Albuterol
- Drugs that are poorly soluble in alkaline pH.
- Drugs that have a narrow window for absorption, i.e., Drugs that are absorbed mainly from the proximal part of small intestine.
Example: Riboflavin, Levodopa.
- Drugs that are absorbed rapidly from the GI tract.
Example: Amoxicillin.
NEED OF GASTRORETENTIVE DRUG DELIVERY SYSTEMS
Oral dosage forms pose low bioavailability problems because of their fast gastric transition from the stomach, particularly in case of drugs that are less soluble at an alkaline pH of the intestine. Also the drugs that produce their local action in the stomach get quickly emptied and do not get sufficient residence time in the stomach. Therefore, frequency of dose administration in such condition is increased. To avoid such problem floating drug delivery system has been developed.
APPROACHES FOR GRDDS [5, 13-15]
The different approaches established for formulating dosage form to produce a satisfactory gastric retention and release within gastric region, are as follows:
- High-density system
- Floating system
- Hydrodynamically balanced system
- Gas-generating system
- Raft-forming system
- Low-density system
- Expandable system
- Super porous hydrogels
- Mucoadhesive or bioadhesive system
- Magnetic system
- Self-unfolding systems
STOMACH SPECIFIC FLOATING DRUG DELIVERY SYSTEM [14]
Stomach specific FDDS has a bulk density lesser than gastric fluids and therefore remain buoyant in the stomach without altering the gastric emptying rate for a longer period of time. However, the system is float on gastric contents, the drug is released gradually at a preferred rate from the system. After releasing drug, the residual system is emptied from stomach. It results in an increased gastric residence time and a better control of variations in plasma drug concentration. The floating SRDF present most of the characteristics of hydrophilic matrices and called ‘hydrodynamically balanced systems’ (HBS) as they are able to preserve their low apparent density, however the polymer hydrates and builds a gelled barrier on the outer surface. The drug is released gradually from the swollen matrix, as in case of conventional hydrophilic matrices. These forms are anticipated to remain buoyant (3-4 hrs) on the gastric contents without altering the intrinsic rate of emptying because their bulk density is lesser than that of the gastric contents. Amongst the different hydrocolloids suggested for floating form formulations, cellulose ether polymers are common, particularly hydroxyl propyl methyl cellulose (HPMC). A fatty material with bulk density <1 may be added to the formulation to increase the buoyancy and reduce the water intake rate.
Similar to formulation studies, research has been take on humans and animals to evaluate intragastric retention performances of floating forms. These calculations were realized either directly by gamma scintigraphic and X-ray monitoring of the form transit in the GIT or indirectly by pharmacokinetic studies with drug tracer.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MECHANISM OF FLOATING DRUG DELIVERY SYSTEM [8]
While the system is floating on the stomach, the drug is released gradually at the desired rate from system. After releasing drug, the residual system is flattened from the stomach besides a minimal gastric content needed to permit suitable attainment of the buoyancy retention principle, a minimal level of floating force (F) is also essential to retain dosage form constantly buoyant on surface of the meal. The apparatus used to measure the floating force kinetics operates by measuring continuously the force equivalent to F (as a function of time) that is mandatory to main submerged object. If F is on the higher positive side, object floats better. The apparatus helps in optimizing floating drug delivery system with respect to stability and durability of floating forces formed in order to stop the problems of unexpected intragastric buoyancy capability variations (Figure 4).
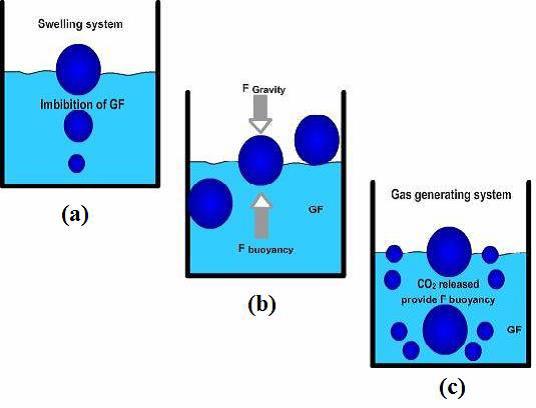
Figure 4. Mechanism of floating system, GF = Gastric Fluid [16]
F = F Buoyancy - F Gravity
= (Df-Ds) × gv --- (1)
Where; F = Total vertical force, Df = Fluid density, Ds = Object density, g = Acceleration due to gravity, v = Volume
CLASSIFICATION OF FDDS BASED ON THE MECHANISM OF BUOYANCY [17-20]
A. Single Unit Floating Dosage Systems
a) Effervescent Systems
b) Noneffervescent Systems
B. Multiple Unit Floating Dosage Systems
a) Noneffervescent Systems
b) Effervescent Systems
c) Hollow Microspheres
C. Raft-Forming Systems
[A] SINGLE UNIT FLOATING DOSAGE SYSTEMS
a) Effervescent Systems (Gas-generating Systems):
These systems used matrices prepared with swellable polymers such as polysaccharides like chitosan, HPMC, citric acid and tartaric acid, effervescent components like sodium bicarbonate or chambers containing liquid that gasifies at body temperature. The optimum stoichiometric ratio of sodium bicarbonate and citric acid for gas generation is described to be 1:0.76. The commonest approach for preparing these systems contains resin beads loaded with bicarbonate and coated with ethyl cellulose. The coating, that is insoluble but permeable, allows permeation of water. So, Co2 is released, causing the beads to float in stomach.
Excipients used in these systems include Carbopol®, polyacrylate polymers, HPMC, polyvinyl acetate, sodium alginate, polyethylene oxide, agar, polycarbonates and calcium chloride.
b) Non-effervescent Systems:
These types of systems, after swallowing, swell unrestrained via imbibition of gastric fluid to a level that it stops their exit from stomach. The systems can be discussed as ‘plug-type systems’ as they have a tendency to persist lodged near pyloric sphincter. One of the formulation approaches of such dosage forms contains the mixing of drug and gel that swells in contact with GI fluid after oral administration and keeps a relative integrity of shape and a bulk density <1 within outer gelatinous barrier. The air surrounded by swollen polymer discusses buoyancy to these dosage forms. E.g., Colloidal gel barrier, micro porous compartment system, alginate beads and hollow microspheres.
Additional type is fluid-filled floating chamber that comprises addition of a gas-filled floatation chamber into micro porous component that houses a drug reservoir. Apertures are present along the top and bottom walls through which the GI fluid enters to dissolve drug. Other two walls in contact with fluid are sealed therefore the undissolved drug stay therein. The fluid present could be air, under partial vacuum or any other appropriate solid, liquid or gas having an appropriate specific gravity and an inert behaviour. The device is of swallowable size, remains afloat in stomach for longer period of time, and after the complete release shell disintegrates, passes off to intestine and eliminated (Figure 5).

Figure 5. Gas filled floatation chamber [16]
[B] MULTIPLE UNIT FLOATING DOSAGE SYSTEMS:
In spite of intensive research and development in the area of hydrodynamically balanced system and other floating tablets, these systems undergo a significant drawback of the high variability of GI transit time, when orally administered, due to their all or nothing gastric emptying nature. With the intension to solve the above difficulty, multiple unit floating systems were established, that decrease the inter-subject variability in absorption and reduce the chance of dose dumping. Reports are obtained in the development of both effervescent and non-effervescent multiple unit systems. Much research has been intensive and the scientists are still discovering the field of hollow microspheres, able to float on gastric fluid and having better GI retention properties.
a) Non-effervescent Systems:
No far report was found in literature on non-effervescent multiple unit systems, when compared with effervescent systems. Though, few workers have described the probability of developing such system comprising Indomethacin, by using chitosan as polymeric excipient. A multiple unit hydrodynamically balanced system comprising Indomethacin as a model drug produced by extrusion method is described. A mixture of drug, acetic acid and chitosan is extruded through a needle, and extrudate is cut and dried. Chitosan hydrates floats in acidic media, and required drug release could be achieved by altering drug-polymer ratio.
b) Effervescent Systems (Gas-generating Systems):
Ikura et al.described sustained release floating granules comprising tetracycline hydrochloride. The granules are the mixture of drug granulates of two stages A and B (A contains 60% of HPMC, 40% of polyacrylic acid and 20% of the drug and B contains 70% of sodium bicarbonate and 30% of tartaric acid). 60% by weight of granules of stage A and 30% by weight of granules of stage B are mixed with a lubricant and packed into capsules. In dissolution media, the capsule shell dissolve and liberate the granules, that showed a floating time of more than 8 hrs and sustained drug release of 80% in about 6.5 hrs. Floating minicapsules of pepstatin that have a diameter of 0.1-0.2 mm has been described by Umezawa.

Figure 6. (A) Multiple-unit oral floating drug delivery system[16]
(B) Mechanism of floatation via Co2 generation [16]
These mini capsules comprise a central core and a coating. The central core comprises of a granule composed of lactose, sodium bicarbonate and a binder that is coated with HPMC. Pepstatin is coated on top of HPMC layer. The system floats due to the release of carbon dioxide in gastric fluid and pepstatin exist in the stomach for longer periods (Figure 6).
c) Hollow Microspheres:
Hollow microspheres are regarded as the most promising buoyant systems, as they’ve exclusive advantages of multiple unit systems and also better floating properties, due to central hollow space inside microsphere. The general methods involved in their preparation contain simple solvent evaporation, solvent diffusion & evaporation. The drug release and better floating properties generally depend on plasticizer, type of polymer and solvents employed for preparation. Polymers like cellulose acetate and Eudragit were used in preparation of hollow microspheres, and drug release can be moderated by enhancing polymer quantity and polymer plasticizer ratio.
[C] RAFT FORMING SYSTEMS:
These systems have established much attention for delivery of antacids and drug delivery for GI disorders and infections. The mechanism complied in the raft formation contains the development of viscous cohesive gel in contact with GI fluids, in which each portion of liquid swells forming a continuous layer known as raft. The raft floats on gastric fluids due to low bulk density produced by the development of Co2. Generally, the system comprises a gel forming agent and alkaline carbonates or bicarbonates liable for the development of Co2 to make the system fewer dense and float on the GI fluids. Jorgen et al. defined an antacid raft forming floating system. The system comprises gel forming agent, acid neutralizer and sodium bicarbonate that forms a foaming sodium alginate gel when in contact with GI fluids. The raft thus formed, floats on GI fluids and stops the reflux of the GI contents into the esophagus by acting as a barrier amongst the esophagus and stomach.
Table 1: COMPARISONS BETWEEN CONVENTIONAL AND GASTRORETENTIVE DRUG DELIVERY SYSTEM [22]:
|
Parameters |
Gastroretentive Drug Delivery System |
Conventional Drug Delivery System |
|
Risk of toxicity |
Lower |
Higher |
|
Patient compliance |
High compliance level |
Less compliance level |
|
Dose dumping |
High risk |
No risk |
|
Drugs |
Beneficial for drugs: |
Not beneficial for drugs: |
|
|
That have rapid GI absorption |
That have low GI absorption |
|
Degrade in colon |
Degrade in colon |
|
|
That show local action in the stomach |
That show local action in the stomach |
Table 2
LIST OF DRUGS FORMULATED AS SINGLE AND MULTIPLE UNIT FORMS OF FLOATING DRUG DELIVERY SYSTEMS [16]:
|
DOSAGE FORMS |
DRUGS |
|
Floating microspheres |
Aspirin, p-nitroaniline, Griseofulvin, Ketoprofen, Ibuprofen, Verapamil and Terfinadine |
|
Floating Films |
Cinnarizine, p-Aminobenzoic acid, Prednisolone and Piretanide |
|
Floating tablets and Pills |
Acetaminophen, Isosorbide mononitrate, Ampicillin, Atenolol, Theophylline, p-aminobenzoic acid, Aspirin, Verapamil hydrochloride, Sotalol |
|
Floating Capsules |
Diazepam, Furosemide, Misoprostol, L-Dopa and Benserazide, Pepstatin, Verapamil HCl and Nicardipine |
|
Floating powders |
Riboflavin, Phosphate, Sotalol and Theophylline |
|
Floating granules |
Diclofenac sodium, Indomethacin, Prednisolone, Cinnarizine, Diltiazem, Fluorouracil and Isosorbide mononitrate |
POLYMERS AND OTHER INGREDIENTS USED IN THE FORMULATIONS OF GASTRORETENTIVE DOSAGE FORMS
- Hydrocolloids (20%-75%): They may be anionic, synthetics or non-ionic like modified cellulose derivatives, hydrophilic gums. E.g., Acacia, Agar, Chitosan, Casein, Bentonite, Veegum, Gellan gum, Sodium CMC, Pectin, MC, HPC, HPMC K4 M, HPMC K15 M, Eudragit S100, Calcium alginate, HPMC K100 M, Eudragit, Propylene foam, Ethyl cellulose, Polyethylene oxide, β Cyclodextrin, Polyethylene glycol, Sodium alginate, PVA, Polycarbonate, Carbopol, PVP, E4 M, Acrylic polymer and CP 934P.
- Inert fatty materials (5%-75%): Edible, inert fatty materials that have specific gravity <1 can be used to reduce the hydrophilic property of the formulation and therefore increase buoyancy. E.g., Fatty acids, Beeswax, Gelucires® 39/01 & 43/01, long chain fatty alcohols, etc.
- Effervescent agents:Citric acid, Sodium bicarbonate, Tartaric acid, Citroglycine, Di-Sodium Glycine Carbonate, etc.
- Release rate accelerants (5%-60%): Lactose, Mannitol, etc.
- Release rate retardants (5%-60%): Dicalcium phosphate, Talc, Magnesium stearate, etc.
- Low density material: Polypropylene foam powder.
- Buoyancy increasing agents (up to 80%):Ethyl cellulose.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CHARACTERIZATION OF GASTRORETENTIVE DOSAGE FORMS [21, 22]
1) Measurement of buoyancy capabilities of the GRDF:
The floating behaviour is estimated with resultant weight measurements. The high molecular weight polymers with slow rate of hydration has greater floating behavior and it is observing more in the simulated meal medium compared to deionized water.
2) Floating time and dissolution:
Floating time measurement test is generally carried out in stimulated gastric fluid or in 0.1 N HCl kept at 37 ºC. It is carried out by using USP dissolution apparatus comprising 900 ml of 0.1 N HCl as dissolution medium. The time occupied by the dosage form to float is called floating lag time. The time for which dosage form float is called as the floating time.
3) Drug loading, particle size analysis and surface characterization:
In case of floating beads and microspheres; drug loading is evaluated by crushing precisely weighed sample of microspheres/beads in a mortar and added to the suitable dissolution medium that is then centrifuged, filtered and analyzed by several analytical techniques such as spectrophotometry.

The particle size and size distribution of microspheres or beads are determined in dry state by using optical microscopy technique. The cross-sectional and external morphology is done by SEM.
4) In-vitro release study:
It is performed to provide the amount of drug that is released at a definite time period. It is carried out by synthetic membrane, Franz diffusion cell system and also by different types of dissolution apparatus.
5) X-Ray/Gamma Scintigraphy:
It locates dosage form in GIT and by that one can predict and correlate the GET and the passage of dosage form in gastrointestinal tract. The inclusion of radio opaque material in a solid dosage form allows it to be envisioned by X- rays. Also, the inclusion of a γ-emitting radionuclide in formulation permits indirect external observation using γ-camera / scintiscanner. In case of γ-scintigraphy, the γ-rays emitted by radionuclide are focused on camera that helps to monitor the position of dosage form in the gastrointestinal tract.
6)Swelling study:
It is performed to calculate molecular parameters of swollen polymers.It is determined by using optical microscopy, dissolution apparatus and other sophisticated techniques that include Confocal laser scanning microscopy, 1H NMR imaging, Light scattering imaging, Cryogenic SEM, etc. The swelling study by using dissolution apparatus is calculated by following formula:

7)Determination of the drug content:
% Drug content provides how much amount of drug which is present in formulation. It must not exceed the limits acquired by standard monographs. It is determined by using HPTLC, HPLC, Inductively Coupled Plasma Atomic Emission Spectrometer, Micro titrimetric methods, Near infrared spectroscopy, etc.
8) Percentage entrapment efficiency:
% Entrapment efficiency is dependable for quantifying phase distribution of drug in prepared formulation. It is calculated by using pressure ultra-filtration, ultra-centrifugation or micro dialysis method.
APPLICATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEMS
1) Enhanced Bioavailability:
Bioavailability of riboflavin controlled release gastroretentive dosage forms is significantly improved compared to administration of non-controlled release gastroretentive dosage forms polymeric formulations. There are numerous different procedures, related to absorption and transit of drug in the GIT, which act concomitantly to impact the magnitude of drug absorption. (Cook JD et al., 1990)
2) Sustained Drug Delivery:
Oral controlled release formulations are faced with problems like GRT in the gastrointestinal tract. HBS systems can be used to overcome the problems that can remain in stomach for prolonged period of time and have bulk density <1 as a result of which they can float on the GI contents. The systems are comparatively large in size & passing from pyloric opening is prohibited.
3) Site Specific Drug Delivery Systems:
These systems are frequently beneficial for drugs which are especially absorbed from stomach or the proximal part of small intestine. The controlled/slow delivery of drug to the stomach offers adequate local therapeutic levels and limits the systemic exposure to drug. This decreases side effects that are produced by drug in blood circulation. Also, the extended gastric availability of a site directed delivery system may reduce the frequency of dosing. E.g., Furosemide and Riboflavin. (Menon A et al., 1994)
4) Absorption Enhancement:
Drugs that are having poor bioavailability due to site specific absorption from upper part of the gastrointestinal tract are potential candidates to be formulated as FDDS, thus maximizing their absorption. (Rouge N et al., 1998)
5) Minimized adverse activity at the colon:
Retention of drug in HBS systems at the stomach reduces the amount of drug that extents the colon. Hence, undesirable activities of drug in the colon can be prohibited. This pharmacodynamics aspect offers the rationale for gastroretentive dosage form for beta lactam antibiotics which are absorbed only from small intestine, and whose presence in colon leads to the growth of microorganism resistance.
6) Reduced fluctuations of drug concentration:
Constant input of drug following controlled release GRDF administration produces blood drug concentrations within narrower range related to immediate release dosage forms. Hence, fluctuations in drug effects are reduced and concentration dependent adverse effects that are related with peak concentrations can be prohibited.
Refer table 3 for patents on gastroretentive dosage forms.
Table 3
PATENTS ON GASTRORETENTIVE DOSAGE FORMS
|
Sr. No. |
Patent No. |
Type of Formulation |
References |
|
1 |
EP 2765998 A1 |
A gastroretentive dosage system and process of preparation thereof |
Varinder Kumaret al., 2014 |
|
2 |
WO 2012007159 A3 |
Novel gastro-retentive dosage forms |
Ramesh Sesha, 2012 |
|
3 |
US 20110182988 A1 |
Gastroretentive pharmaceutical dosage form |
Viness Pillayet al., 2011 |
|
4 |
WO 2011048494 A2 |
Novel gastroretentive dosage forms of poorly soluble drugs |
Nadav Navon, 2011 |
|
5 |
WO 2011090724 A2 |
Gastroretentive solid oral dosage forms with lipid-based low-density excipient |
Laman Lynn Alaniet al., 2011 |
CONCLUSION
Development of an efficient gastroretentive dosage form for stomach specific drug delivery is an actual challenge. Thus, to produce the preferred gastro retention several methods have been used, out of which, the floating drug delivery system has emerged as the best promising method. These systems provide the benefit of better absorption of drugs that are absorbed from upper part of stomach. Local action of drug is increased as the system rests in stomach for longer time. This leads to less frequent dosing and enhanced efficiency of the treatment. Good stability and better drug release as compared to other conventional dosage forms make such system more reliable. Drug absorption in GIT is a highly variable procedure and prolonging GI retention of the dosage form prolongs the time of drug absorption. Floating drug delivery system promises to be a potential approach for gastric retention. Though there are number of complications to be worked out to achieve extended GI retention, many companies are focusing toward commercializing this method.
REFERENCES
1. Kagan L, Hoffman A; Systems for region selective drug delivery in gastrointestinal tract; biopharmaceutical considerations; Expert Opinion on Drug Delivery; 2008; 5(6); 681-692.
2. Bardonnet P, Faivre V, Pugh W, Piffaretti J, Falson F; Gastroretentive dosage forms, Overview and special case of Helicobacter pylori; Journal of Controlled Release; 2006; 111; 1-18.
3. Deshpande AA, Shah NH, Rhodes CT, Malick W; Development of a novel controlled release system for gastric retention; Pharmaceutical Research; 1997; 14(6); 815‐819.
4. Patil, JM, Hirlekar RS, Gide PS, Kadam VJ; Trends in floating drug delivery system; Journal of Scientific and Industrial Research; 2006; 65; 11-21.
5. Arunachalam A, Karthikeyan M, Konam K, Prasad HP, Sethuraman S, Ashutoshkumar S, Manidipa S; Floating drug delivery systems: A Review; International Journal of Pharmaceutical Science and Research; 2011; 2(1); 76-83.
6. Tortora GJ, Grabowski SR. Principles of Anatomy and Physiology; New York; John Wiley and Sons; 2002.
7. Marinaganti RK, Bonthu S, Nagakanyaka DP, Sheik M; A comprehensive review on gastro retentive drug delivery system; 2013; 3(2); 149-164.
8. Garg S, Sharma S; Gastroretentive Drug Delivery System; Business Briefing: Pharmatech; 2003; 160‐166.
9. Brahmankar DM, Jaiswal SB; Biopharmaceutics and pharmacokinetics a treatise; New Delhi; Vallabh Prakashan; 2003.
10. Patel GM, Patel HR, Patel M; Floating drug delivery system: An innovative approach to prolong gastric retention; Pharmainfo.net 2007; 5(6).
11. Jain NK; Progress in controlled and novel drug delivery system; Delhi; CBS Publishers; 2003.
12. Babu VBM, Khar RK; In vitro and In vivo studies of sustained release floating dosage forms containing salbutamol sulphate; Pharmazie; 1990; 45(4); 268-270.
13. Nasa P, Mahant S, Sharma D; Floating systems: a novel approach towards gastroretentive drug delivery systems; International Journal of Pharmacy and Pharmaceutical Sciences; 2010; 2(3); 2-7.
14. Reddy LH, Murthy RS; Floating dosage systems in drug delivery; Critical reviews in therapeutic drug carrier systems; 2002; 19(6); 553-585.
15. Shah SH, Patel JK, Pundarikakshudu K, Patel NV; An overview of a gastroretentive floating drug delivery system; Asian Journal of Pharmaceutical Sciences; 2009; 4(1); 65-80.
16. Gupta G, Singh A; A Short Review on Stomach Specific Drug Delivery System; International Journal of PharmTech Research; 2012; 4(4); 1527-1545.
17. Rubinstein A, Friend DR; Specific delivery to the gastrointestinal tract; In: Domb AJ, editor; Polymeric Site-Specific Pharmacotherapy; Wiley & Chichester; 1994; 282-283.
18. Ikura, H, Suzuki Y, Nagai T, Machida Y, inventors; Teijin Limited, assignee; Cellulose ether, polyacrylic acid, foaming agent, drug; United States Patent US 4777033 A; 1988 Oct 11.
19. Umezawa H, inventor; Zaidan Hojin Biseibutsu Kagaku Kenkyu Kai, assignee; Pepstatin floating minicapsules; United States Patent US 4101650 A; 1978 Jul 18.
20. Fabrcgas JL, Cucala CG, Pous J; Sites R.A. In vitro testing of an antacid formulation with prolonged gastric residence time; Drug Development and Industrial Pharmacy; 1994; 20; 1199-1212.
21. Agnihotri SA, Jawalkar SS, Aminabhavi TM; Controlled release of cephalexin through gellan gum beads: Effect of formulation parameters on entrapment efficiency, size, and drug release, European Journal of Pharmaceutics and Biopharmaceutics; 2006; 63(3); 249-261.
22. Harries D, Sharma HL; GI transit of potential bioadhesive formulations in man: A scintigraphic study; Journal of Controlled Release; 1990; 12(1); 45-53.









